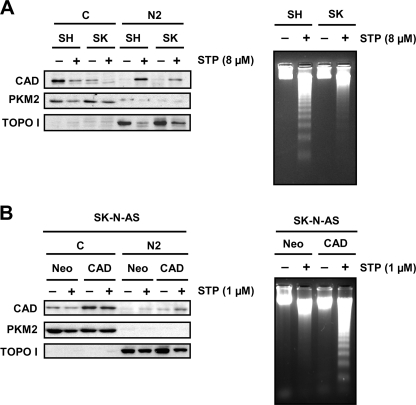
FIGURE 8.
The lack of DNA laddering in STP-treated SK-N-AS cells is not due to a defect in the translocation of DFF40/CAD to the chromatin-enriched fraction but determined by the cytosolic levels of the endonuclease. A, SK-N-AS (SK) and SH-SY5Y (SH) cells were treated (+) or not (−) with 8 μm STP for 6 h. Then cells were fractionated to obtain the cytosolic (C) and the chromatin-enriched (N2) fractions. DFF40/CAD protein was assessed in the cytosolic (C) and the chromatin-enriched fraction (N2). Otherwise, DNA was extracted and analyzed by conventional agarose gel electrophoresis to verify the oligonucleosomal DNA degradation. B, SK-N-AS cells stably transfected with empty pcDNA3 (Neo) or pcDNA3-DFF40/CAD (CAD) plasmids were treated with 1 μm STP (+) or left untreated (−) for 6 h. Cells were fractionated to obtain the cytosolic (C) and the chromatin-enriched (N2) fractions, and DFF40/CAD protein was assessed by Western blot. Alternatively, DNA was extracted and analyzed by conventional agarose gel electrophoresis to analyze the DNA laddering. Note that only DFF40/CAD overexpressing cells display a DNA laddering pattern upon STP treatment. PKM2 (middle panel) and topoisomerase I (TOPO I, lower panel) were run as cytosolic and nuclear protein markers, respectively.