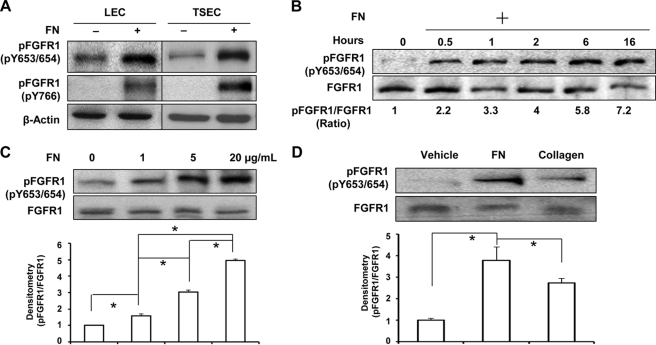
FIGURE 1.
Fibronectin induces FGFR1 phosphorylation. A, two liver endothelial cell types, human LEC and murine-derived TSEC, were serum-starved overnight and then plated on 5 μg/ml FN- or PBS-precoated dishes in basal medium. Total cellular protein was extracted, and phospho-FGFR1 at tyrosines 653/654 and 766 was probed by Western blot with β-actin used as a loading control. The blots are representative of three independent experiments. B, serum-starved TSECs were plated on 5 μg/ml FN-coated dishes for varying duration (0, 0.5, 1, 2, 6, and 16 h). Phosphorylation of FGFR1 at Tyr-653/654 and total FGFR1 were evaluated by Western blot. Densitometric results of the pFGFR1/FGFR1 ratio are shown below the representative blots, which were normalized by the ratio at time zero and compiled as the mean from three independent experiments. C, LECs were serum-starved and replated on FN at different concentrations (0, 1, 5, and 20 μg/ml) in basal medium for at least 4 h. Phosphorylation of FGFR1 at Tyr-653/654 and total FGFR1 were evaluated. The densitometric result of the pFGFR1/FGFR1 ratio is shown in the histogram, normalized by the ratio in the FN0 group (n = 3; *, p < 0.05 between depicted groups, using one-way ANOVA with post hoc test). D, serum-starved TSECs were seeded either on 10 μg/ml collagen I, 5 μg/ml FN-coated dishes or on a PBS-coated dish as a control in basal medium for 16 h. FGFR1 phosphorylation at Tyr-653/654 and total FGFR1 were evaluated by Western blot. Densitometric results of the pFGFR1/FGFR1 ratio are shown in the histogram below the representative blot, normalized by the ratio of the control group (n = 3; *, p < 0.05). Error bars, S.E.