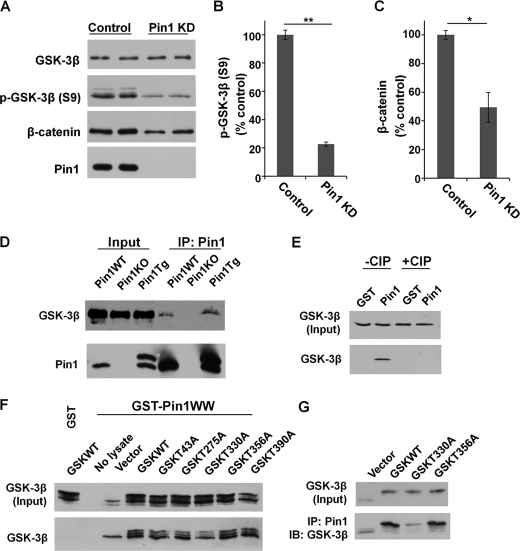
FIGURE 1.
Pin1 binds to phosphorylated Thr-330-P motif in GSK3β. A–C, Pin1 knockdown (Pin1 KD) reduced p-GSK-3β (S9), leading to higher GSK3β activity and a higher rate of β-catenin degradation. Error bars indicate S.D. D, Co-IP confirmed the endogenous interaction of GSK3β and Pin1 in Pin1-WT and Pin1-Tg mouse brains. E, Pin1 binding to GSK3β is dependent on phosphorylation and is abolished after CIP treatment, as assayed by GST-Pin1 pulldown assay. F and G, Pin1 binding to GSK3β point mutants was assayed by GST-Pin1 pulldown (F) and Co-IP (G). *, p < 0.05; **, p < 0.005. IB, immunoblotting.