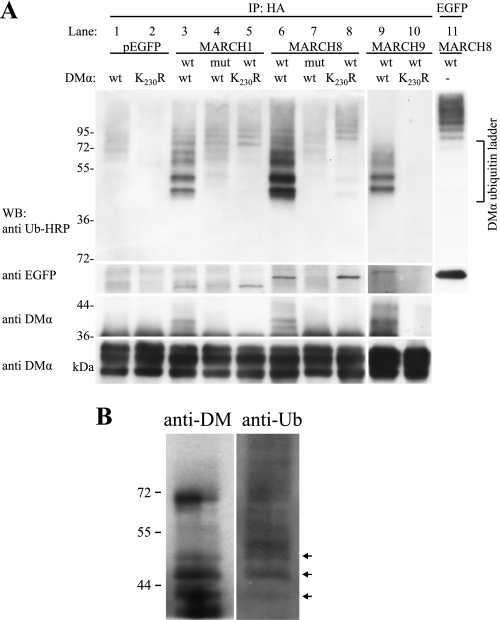
FIGURE 3.
HLA-DM is modified by polyubiquitination. DMα/DMβHA and DMα-K230R/DMβHA constructs were transfected into HEK-293T cells together with wt or mutant (mut) constructs of EGFP-MARCH1, EGFP-MARCH8, EGFP-MARCH9, or pEGFP. After 24 h, DMαβHA was immunoprecipitated (IP) with nondepleting quantities of anti-HA mAb and products analyzed by SDS-PAGE and Western blotting (WB). A, ubiquitinated molecules associated with immunoprecipitated DMαβHA were detected using anti-ubiquitin antibody P4D1-HRP (upper, lanes 1–10). Products corresponding in size to ubiquitinated DMα are labeled (DMα ubiquitin ladder). Ubiquitinated EGFP-MARCH8 is shown in lane 11. Products above the DMα ubiquitin ladder likely represent ubiquitinated EGFP-MARCH proteins. Co-immunoprecipitating EGFP-MARCH proteins detected with anti-GFP antibody are shown in panel WB:anti EGFP. Anti-DMα mAb 5C1 was used to detect both ubiquitinated (panel WB:anti DMα upper, lanes 3, 6, and 9, arrowheads) and nonubiquitinated (panel WB:anti DMα lower) DMα products. Long and short exposure times were required for panels WB:anti DMα upper and lower, respectively. Data are representative of three independent experiments. Lanes 9 and 10 were run on a separate gel. B, endogenously expressed DMαβ was precipitated from membrane fractions of Raji cells with anti-DMαβ mAb immobilized to protein G beads and analyzed by SDS-PAGE and Western blotting. The blot was first probed with anti-ubiquitin-HRP and then with anti-DMα antibody. Arrows denote bands with the same migration properties. Data are representative of three independent experiments.