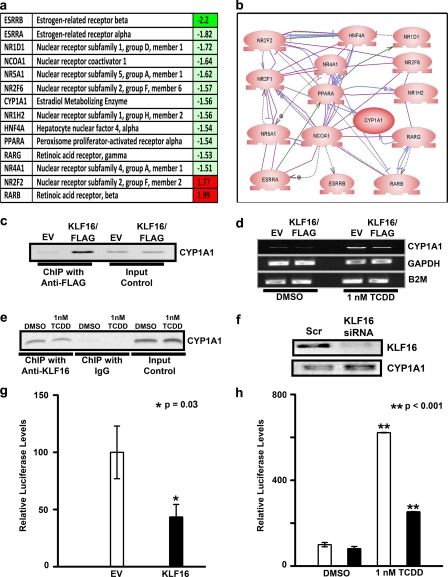
FIGURE 2.
KLF16 functions as transcription factor involved in the regulation of metabolic and endocrine gene expression in uterine cells. a, selected identifiers for KLF16-regulated genes. Most of these genes (80%) are down-regulated (green) by this transcription factor, which is congruent with the predominant gene-silencing activity of KLF16. Notably, from these genes, 22% participate in estrogen metabolism. b, semantic-based pathway reconstruction algorithms integrate most of the genes found regulated by KLF16 in the PCR microarray into a seamless pathway, showing active interaction among its nodes. KLF16 regulated the expression of several genes connected with metabolism of sex steroid as well as environmental contaminant toxins in endometrial cells. c, chromatin immunoprecipitation was performed on uterine cells transfected with either pCMV/FLAG- or pcDNA3HIS-KLF16 or corresponding empty vectors. Anti-FLAG or anti-HIS antibodies were used for immunoprecipitation, respectively. Shown is a representative sample where FLAG-KLF16 but not empty vector bound a 249-bp CYP1A1 genomic element containing the BTE (−55 to −41) in uterine cells. d, uterine cells were transfected with either FLAG-KLF16 or corresponding empty vector. The cells were treated with 1 nm dioxin to induce CYP1A1 expression. Total RNA was extracted at 48 h, and RT-PCR was done. KLF16 repressed TCDD induction of CYP1A1 mRNA expression by 50% (p < 0.05) compared with the corresponding empty vector. Housekeeping genes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β2 microglobulin (B2M) were used as loading controls. e, ChIP was performed for endogenous KLF16 in the presence or absence of 1 nm dioxin. Positive amplification of the CYP1A1 promoter demonstrates that KLF16 binds this genomic element described above both in the presence and absence of dioxin. f, uterine cells were transfected with KLF16 siRNA to knock down endogenous KLF16 levels, which was confirmed by Western blot using anti-KLF16 (upper panel). CYP1A1 mRNA levels were increased 2-fold in cells treated with KLF16 siRNA (lower panel). g, uterine cells were transfected with 7.5 μg of FLAG-KLF16 or corresponding empty vector and 2.5 μg of CYP1A1 promoter-luciferase constructs containing the BTE (−55 to −41). Results of luciferase-reporter assays normalized to protein expression revealed that KLF16 significantly repressed CYP1A1-luciferase activity compared with empty vector control (46%; *, p = 0.03). h, CYP1A1 enzymatic activity was measured using a well characterized CYP substrate-reporter assay. Uterine cells were transfected with either KLF16 (black bars) or empty vector control (white bars) and subsequently treated with either 1 nm TCDD or DMSO control. A luciferin-derived CYP1A1 substrate was added to the medium and enzyme activity determined by reporter activity. CYP1A1 enzymatic activity was significantly inhibited by KLF16 (60%; **, p < 0.001) in TCDD treated cells.