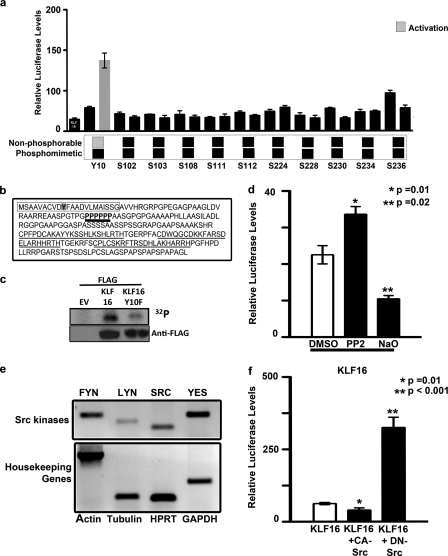
FIGURE 7.
Gene silencing activity KLF16 is regulated by tyrosine modifications. a, uterine cells were cotransfected with constructs from a library of in vitro mutagenized KLF16-Gal4 proteins where individual serine, threonine, and tyrosine residues were replaced with either alanine (phenylalanine for tyrosine) or aspartic acid as indicated. The Gal4-luciferase reporter system was used to evaluate the transcriptional role of each mutant. Whereas all of the serine/threonine mutants repressed reporter expression analogous to WT-KLF16, the KLF16Y10F mutant (where tyrosine 10 in the WT protein was replaced by nonphosphorylatable phenylalanine) reversed repression. b, N terminus of KLF16 contains a proline-rich domain (boldface type, black underline). Also indicated are the Sin3-interacting domain (box) containing tyrosine 10 (shaded), and the three zinc fingers (gray underline). The proline-rich domain is located close to the KLF16-SID. c, to detect in vivo phosphorylation of KLF16, Ishikawa cells were transfected with EV, FLAG-KLF16, or FLAG-KLF16Y10F and subsequently labeled with [32P]orthophosphate. Phosphorylation of KLF16 was detected by immunoprecipitation using anti-FLAG followed by exposure to autoradiography film. d, uterine cells transfected with 10 μg of FLAG-KLF16 were treated with either Src tyrosine kinase-specific inhibitor PP2 or tyrosine phosphorylase-specific inhibitor sodium orthovanadate. PP2 reversed reporter repression, compared with vehicle-treated control (*, p = 0.01). In contrast, sodium orthovanadate augmented repression (**, p = 0.02). e, RT-PCR was used to evaluate the expression of Src family members in uterine cells. Four out of eight were expressed: FYN, LYN, SRC, and YES kinases. Human β-actin, β-tubulin, HPRT, and GAPDH were used as internal controls. f, luciferase reporter assays were performed in uterine cells transfected with either 5 μg of FLAG-KLF16 or corresponding EV and 5 μg of either a constitutively active or dominant negative (kinase-inactive) SRC kinase construct along with 2.5 μg of 6×-BTE-luciferase reporter construct. Luciferase levels normalized to protein concentration and parent vector showed that whereas luciferase reporter was repressed by KLF16 and constitutively active SRC (*, p = 0.01), repression was reversed when KLF16 was cotransfected with kinase inactive DN-SRC (**, p < 0.001) indicating the role of Src kinase in post-translational regulation of KLF16.