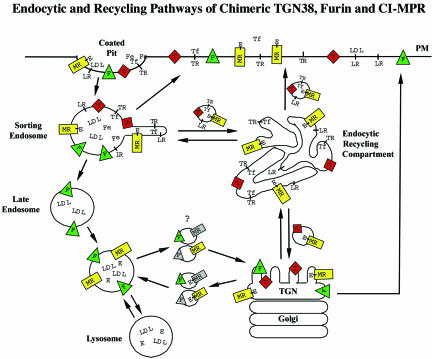
Figure 10.
Schematic model of CI-MPR trafficking in CHO cells. The model compares the postendocytic itineraries of the CI-MPR (MR) and its enzyme ligands (E) with the transferrin receptor (TR), transferrin (Tf) with or without iron (Fe), the LDL receptor (LR), LDL, furin (F), and TGN38 (T). All of the membrane proteins concentrate into clathrin-coated pits, and the initial delivery site is sorting endosomes. Most of the membrane proteins, including CI-MPR, rapidly exit this compartment and are either returned directly to the plasma membrane (PM) or are transported to the ERC. Furin is retained in the sorting endosome as it begins to mature into a late endosome, and furin reaches the Golgi via the late endosomes. From the ERC, essentially all of the transferrin receptors recycle to the cell surface, and ∼85% of the internalized TGN38 and CI-MPR also return to the cell surface. The remaining TGN38 and CI-MPR are delivered to the trans-Golgi. The CI-MPR traffics from the trans-Golgi to late endosomes, where the enzyme ligands dissociate as a consequence of exposure to a sufficiently acidic pH. Furin and the empty CI-MPR return to the trans-Golgi. It is not known if furin and CI-MPR share the same transport vesicle as they shuttle between late endosomes and the trans-Golgi. A portion of all of the molecules in the trans-Golgi is delivered to the cell surface.