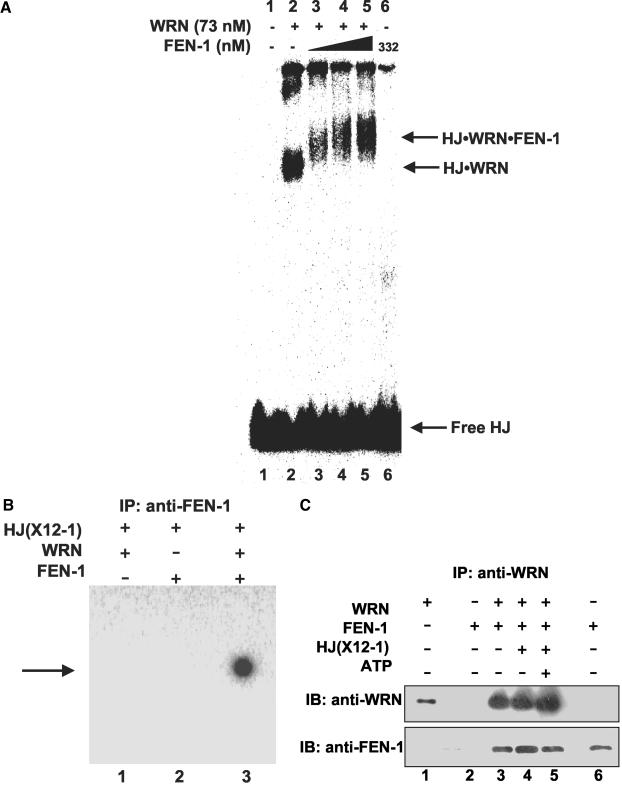
Figure 8.
WRN recruits FEN-1 to the Holliday junction. (A) WRN-HJ complex is super-shifted by FEN-1. Binding mixtures (20 μl) containing 25 fmol of HJ(X12-1), 1 mM ATPγS, WRN (73 nM), and FEN-1 (116, 174, and 332 nM, lanes 3, 4 and 5, respectively) were incubated at 24°C for 20 min followed by 0.25% glutaraldehyde cross-linking. Protein-DNA complexes were analyzed on nondenaturing 5% polyacrylamide gels. A phosphorimage of a typical gel is shown. (B) Identification of FEN-1 in the super-shifted protein-HJ complex. HJ(X12-1) was incubated with WRN and/or FEN-1 under standard binding conditions. Protein-HJ complexes were UV-cross-linked, incubated with anti-FEN-1 antibody, precipitated with protein-G agarose beads, and electrophoresed on 8% polyacrylamide SDS gels. A phosphorimage of a gel from a typical experiment is shown. (C) Purified WRN and FEN-1 interact directly under HJ resolution reaction conditions. Purified WRN (12 nM; lanes 3, 4, 5) and FEN-1 (29 nM; lanes 2, 3, 4, 5) were incubated in HJ resolution buffer in the absence or presence of HJ(X12-1) (37.5 fmol) and/or ATP (2 mM) as indicated. Binding mixtures were subsequently incubated with anti-WRN antibody and adsorbed to protein-G agarose beads. Beads were extensively washed and bound proteins were eluted and resolved on 8-16% SDS-PAGE. Proteins were transferred to PVDF membranes and probed with either mouse anti-WRN or rabbit anti-FEN-1 antibodies as noted. In both panels, markers for recombinant WRN (50 ng; lane 1) and recombinant FEN-1 (50 ng; lane 6) are shown.