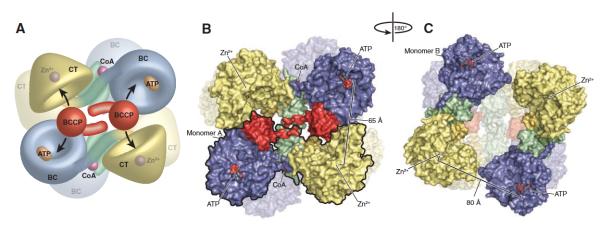
Figure 3.
(A) Model of the R. etli PC tetramer showing the movement of the BCCP domain between neighboring active sites on opposing polypeptide chains. (B) Surface representation of the top face of the tetramer. For clarity, one of the two individual monomers has been outlined in black. The distance between ATP-γ-S in the BC active site and Zn2+ in the CT active site of the opposing polypeptide chain is 65 Å. (C) Surface representation of the bottom face of the tetramer, after a 180° rotation about the y axis. The BCCP domain is disordered in these monomers and could not be modeled. The distance between ATP-γ-S in the BC active site and Zn2+ in the CT active site of the opposing polypeptide chain increases to 80 Å as a result of the altered orientation of the BC domain. Reproduced from St. Maurice et al. [9].