Abstract
The entry of exogenous fibroblast growth factor 2 (FGF-2) to the cytosolic/nuclear compartment was studied and compared with the translocation mechanism used by FGF-1. To differentiate between external and endogenous growth factor, we used FGF-2 modified to contain a farnesylation signal, a CaaX-box. Because farnesylation occurs only in the cytosol and nucleoplasm, farnesylation of exogenous FGF-2-CaaX was taken as evidence that the growth factor had translocated across cellular membranes. We found that FGF-2 translocation occurred in endothelial cells and fibroblasts, which express FGF receptors, and that the efficiency of translocation was increased in the presence of heparin. Concomitantly with translocation, the 18-kDa FGF-2 was N-terminally cleaved to yield a 16-kDa form. Translocation of FGF-2 required PI3-kinase activity but not transport through the Golgi apparatus. Inhibition of endosomal acidification did not prevent translocation, whereas dissipation of the vesicular membrane potential completely blocked it. The data indicate that translocation occurs from intracellular vesicles containing proton pumps and that an electrical potential across the vesicle membrane is required. Translocation of both FGF-1 and FGF-2 occurred during most of G1 but decreased shortly before the G1→S transition. A common mechanism for FGF-1 and FGF-2 translocation into cells is postulated.
INTRODUCTION
Fibroblast growth factor 2 (FGF-2) belongs to the 23-member FGF family of signaling polypeptides, which is characterized by a core region of highly conserved sequence and structure (Mason, 1994; Ornitz and Itoh, 2001). FGF-2 mediates a variety of biological responses involving cell growth and proliferation, migration, and differentiation.
FGF-2 exists as several molecular isoforms translated from a common mRNA by the use of alternative initiation codons (Florkiewicz and Sommer, 1989). The 18-kDa isoform is found both in the cytoplasm and nucleus (Renko et al., 1990) and can be exported out of cells by a mechanism that bypasses the classical ER/Golgi pathway (Florkiewicz et al., 1995). External FGF-2 can interact with cell surface heparans and with high-affinity transmembrane receptors containing an intracellular split tyrosine kinase domain (Powers et al., 2000), which results in the activation of downstream effectors such as phospholipase Cγ and the MAP-kinase pathways.
High-molecular-weight isoforms of FGF-2 (22, 22.5, 24, and 34 kDa) contain N-terminal nuclear localization signals (Quarto et al., 1991; Arnaud et al., 1999), which confer their exclusively nuclear localization.
The 18-kDa isoform of FGF-2 has been shown to interact with some intracellular proteins including protein kinase CKII (Bonnet et al., 1996), ribosomal protein L6/TAXREB107 (Shen et al., 1998), the nuclear protein FIF (Van den Berghe et al., 2000), and the cytoplasmic translokin (Bossard et al., 2003), indicating that the growth factor may act in a dual mode, i.e., by interaction with cell-surface receptors and with cytosolic/nuclear targets. Such dual mode of action has been earlier proposed for FGF-1 (Imamura et al., 1990; Wiȩdlocha et al., 1994), a protein closely related to FGF-2.
Accumulating evidence indicates that exogenous FGF-1 is capable of reaching the cytosol and the nucleus of target cells (Olsnes et al., 2003) and that this may be required for mitogenic response, at least in certain cell types (Wiȩdlocha et al., 1996). The mechanism of FGF-1 translocation has been elucidated to some extent, indicating the requirement of PI3 kinase activity (Klingenberg at al., 2000) and of vesicular transmembrane potential (Malecki et al., 2002).
FGF-1 and FGF-2 share many biological properties but have clearly different functions. Thus, the two growth factors are expressed to very different extent in different tissues and they play different roles during differentiation (Szebenyi and Fallon, 1999). In NIH/3T3 cells FGF-1 is considerably less efficient in inducing the FGF inducible response element (FiRE) than FGF-2 (Jaakkola et al., 1998). Translokin is a protein that interacts with FGF-2 during translocation but it does not interact with FGF-1 (Bossard et al., 2003). Also FIF (FGF-2-interacting factor), a nuclear putative antiapoptotic factor, binds FGF-2 but not FGF-1 (Van den Berghe et al., 2000). FGF-1 binds well to all four FGF receptors and to their different splicing variants, whereas FGF-2 binds weakly to FGF receptor 2 containing a certain splicing variants in the second half of the third immunoglobulin-like loop, also referred to as keratinocyte growth factor receptor (Dell and Williams, 1992; Miki et al., 1992; Yayon et al., 1992). In spite of these differences we decided to test the possibility that the two growth factors could use a common mechanism for translocation to the cytosol and nucleus.
Earlier studies have suggested that external FGF-2 can be transported to the nucleus of target cells (Bouche et al., 1987; Baldin et al., 1990), indicating that exogenous growth factor is able to cross cellular membranes. A problem with such studies is the possibility that externally added growth factor may induce expression of endogenous FGF-2 (Hurley et al., 1994; Peng et al., 2001; Cowan et al., 2003) with its subsequent transport to the nucleus. This could then erroneously be interpreted as external growth factor being translocated to the nucleus. Another problem in studies of protein translocation is to demonstrate that the protein is indeed in the cytosol (or nucleus) rather than in membrane-bound compartments where it may be bound to receptors that are associated with cytoskeletal elements that cosediment with the nuclei.
One way to overcome these problems is to modify the added growth factor such that it can be enzymatically labeled by enzymes that are present only in the cytosol and/or the nucleus. We have previously developed such a method based on farnesylation of proteins modified to contain a C-terminal farnesylation signal, a CaaX-box. Because farnesyl transferase is only found in the cytosol (Reiss et al., 1990) and nucleus (Sinensky et al., 1994), farnesylation of an exogenous CaaX-containing protein will indicate that it has crossed cellular membranes. This strategy was used successfully to demonstrate translocation to cytosol of FGF-1 (Wiȩdlocha et al., 1995) and diphtheria toxin (Falnes et al., 1995). We have used the same approach in studies involving FGF-2 translocation.
MATERIALS AND METHODS
Materials
[14C]mevalonolactone (58 mCi/mmol) and [3H]farnesyl pyrophosphate (21.5 Ci/mmol) were from NEN Life Science Products (Boston, MA), [3H]thymidine and [35S]methionine from Amersham (Piscataway, NJ), heparin-Sepharose and protein A-Sepharose from Pharmacia Biotech (Piscataway, NJ), protease inhibitors cocktail Complete from Roche (Indianapolis, IN), lovastatin from Merck, FGF-2 wt from R&D Systems (Minneapolis, MN), bovine serum albumin (BSA) and pMal-C2 expression and purification system were from New England BioLabs (Beverly, MA), and other chemicals were from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal antiphospho-p44/42 MAP-kinase and rabbit polyclonal anti-p44/42 MAP-kinase were from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal anti FGF-2 was from Santa Cruz Biotechnology (Santa Cruz, CA).
Buffers and Media
Lysis buffer: 10 mM Na-phosphate, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1% Triton X-100 and protease inhibitors cocktail. Dilution buffer: 50 mM Na-phosphate, pH 7.2, 150 mM NaCl, 0.1% sodium azide, 20% glycerol, 10 U/ml heparin, and 0.1 mg/ml BSA. Serum-starvation medium: DMEM + 0.5% (vol/vol) fetal bovine serum.
Expression and Purification of Recombinant Proteins
FGF-1 and FGF-1-CaaX were expressed from pTrc-FGF-1 and pTrc-FGF-1-CaaX and purified as described (Wiȩdlocha et al., 1995). FGF-2-CaaX (18-kDa form) was expressed as MBP fusion protein from pMal-C2-FGF-2-CaaX and purified on amylose resin, according to the manufacturer's protocol. MBPFGF-2-CaaX was treated with factor Xa, blocked with PMSF and used without further purification. The growth factor was diluted to appropriate concentrations in dilution buffer. The cDNA corresponding to full-length FGF-2 (∼18 kDa) and the deletion mutant Δ(3-24) FGF-2 (∼16 kDa) were cloned into plasmid pTriEx-2 (Novagen, Madison, WI). After plasmid linearization, the corresponding proteins were produced in rabbit reticulocyte lysate using in vitro transcription and translation system (Promega, Madison, WI), according to the manufacturer's protocol. During translation 1 μM [35S]methionine and 25 μM aliquots of the other amino acids were used to produce radioactive FGF-2. All proteins obtained in vitro were dialyzed against 20 mM Hepes, pH 7.0, 2 mM CaCl2, 100 mM NaCl. FGF-2 was also expressed in bacteria from pTriEx-2-FGF-2 and purified on heparin-Sepharose as described for FGF-1 (Wiedlocha et al., 1995).
In Vitro Farnesylation
[3H]farnesyl pyrophosphate (0.5 μCi) and 20 ng/ml FGF-2 or FGF-2-CaaX were mixed with 20 μl reticulocyte lysate, containing 5 mM MgCl2, and incubated for 1 h at 37°C. In some cases the incubation mixture contained 50 μM B581. Labeled proteins were treated for 2 h at 4°C with heparin-Sepharose or immunoprecipitated using anti-FGF-2 antibodies adsorbed to protein ASepharose and analyzed by SDS-PAGE (Laemmli, 1970) and fluorography.
Activation of MAP Kinase
Cells were serum-starved for 24 h. The last 2 h of serum-starvation was in fresh medium in the absence or presence of tested compounds. Then cells were treated with 10 U/ml heparin and the given amount of FGF-2 or FGF-2-CaaX for the indicated period of time. Next, the cells were lysed in SDS sample buffer and analyzed by SDS-PAGE and Western blot using anti-p44/42 MAP-kinase antibodies. After stripping, the membrane was reprobed with antibodies against the phosphorylated form of p44/42 MAP-kinase.
Measurements of DNA Synthesis
Cells were serum-starved for 24 h and treated with 10 U/ml heparin and the indicated amount of growth factor for 24 h. During the last 6 h of incubation 1 μCi/ml [3H]thymidine was present in the medium. Next, cells were extracted with 5% trichloroacetic acid (TCA) and the radioactivity incorporated into TCA-insoluble material was measured.
In vivo prenylation was performed essentially as described earlier (Wiȩdlocha et al., 1995; Malecki et al., 2002) with some modifications. Cells were serum-starved for 24 h. For the last 2 h, serum-starvation was continued in fresh medium containing 0.5 μCi/ml [14C]mevalonolactone and 1 μg/ml lovastatin. The tested compounds were added 30 min before the end of the serum starvation. Cells were then treated with 10 U/ml heparin and 100-200 ng/ml growth factor, and the incubation was continued for 5-6 h more. Cells were washed once in PBS, lysed, and sonicated. Lysates were adsorbed onto heparin-Sepharose and analyzed by SDS-PAGE and fluorography.
Intracellular Processing of FGF-2
NIH/3T3 cells were serum-starved for 24 h and treated for 6 h with 10 U/ml heparin and ∼10 ng/ml [35S]methionine-labeled 18-kDa FGF-2. After incubation cells were washed once with 2 M NaCl in 20 mM sodium acetate, pH 4.0 and once with PBS and lysed. The cellular material was sonicated, and the material present in the high-salt/low-pH wash was diluted five times in 50 mM Tris-HCl and adjusted to pH 8.0. Both fractions were adsorbed onto heparin-Sepharose and analyzed by SDS-PAGE and fluorography.
Laser Scanning Confocal Microscopy
FGF-2 and FGF-2-CaaX were labeled directly with Cy3 maleimide (Amersham) or Alexa 488 maleimide (Molecular Probes) following the manufacturer's procedures. NIH/3T3 cells were grown on coverslips and incubated with or without the appropriate inhibitor for 30 min. Fluorophore-labeled FGF-2 and/or FGF-2-CaaX were added, and the cells were further incubated for different time periods. The cells were then washed with PBS and fixed in 3% paraformaldehyde in PBS for 15 min. After washing three times with PBS, the cells were incubated for 5 min with 50 mM NH4Cl. When antibodies were used to visualize structures in the cell, the cells were first permeabilized with 0.1% Triton X-100. The cells were then incubated with primary antibody for 20 min, washed, and incubated with the secondary antibody coupled to rhodamine or Cy3. After staining, the coverslips were mounted in Mowiol and examined with a Leica confocal microscope (Deerfield, IL) or a Zeiss LSM 510 META confocal microscope (Thornwood, NY) in the cases where double labeling experiments were performed. Montages of images were prepared with the use of Adobe Photoshop 7.0 (San Jose, CA).
RESULTS
Properties of FGF-2-CaaX
Addition of the CaaX Motif to FGF-2. Most cells express mRNA for FGF-2, although protein is often not demonstrable (Knee et al., 1994). Therefore, to test if externally added FGF-2 is indeed translocated to the cytosol and nucleus, it is necessary to be able to distinguish the added growth factor from the growth factor synthesized by the cell itself. For this purpose we used a strategy previously used in studies of FGF-1 (Wiȩdlocha et al., 1995).
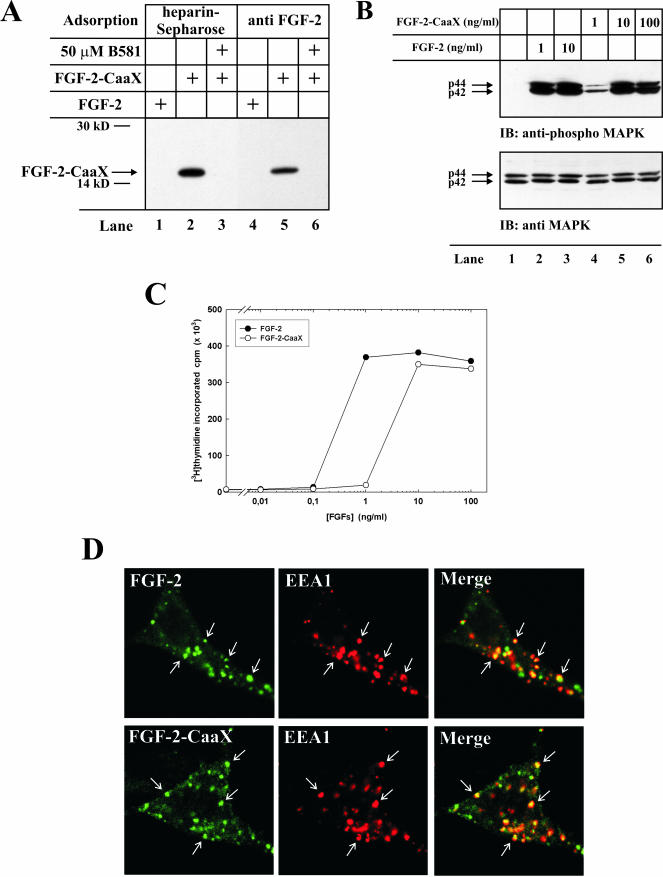
Proteins carrying a C-terminal farnesylation signal, a CaaX box, become farnesylated upon exposure to the cytosol or the nucleoplasm. If the incubation is carried out in the presence of radioactively labeled precursor of the farnesyl group, the protein will be labeled. Because farnesyl transferase is only found in the cytosol (Reiss et al., 1990) and in the nucleus (Sinensky et al., 1994), labeling will indicate that the externally added protein has crossed cellular membranes. We added the CaaX-box of the K-Ras-4B protein (Cys-Val-Ile-Met) onto the C-terminus of the 18-kDa isoform of FGF-2 and expressed it in Escherichia coli, which is unable to farnesylate the protein. To test the activity of the farnesylation signal in the recombinant FGF-2-CaaX, the protein was incubated with [3H]farnesyl pyrophosphate in a reticulocyte lysate and analyzed by SDS-PAGE. A labeled protein band with the expected molecular weight (∼18 kDa) that was adsorbed to heparin-Sepharose was indeed obtained (Figure 1A, lane 2). The protein could be immunoprecipitated using anti-FGF-2 antibodies adsorbed to protein ASepharose (lane 5). There was no labeling when B581, a specific inhibitor of farnesyl transferase (Garcia et al., 1993), was present (lanes 3 and 6). There was also no labeling when FGF-2 without the CaaX box was used (lanes 1 and 4).
Figure 1.
In vitro farnesylation and biological activity of FGF-2-CaaX. (A) Recombinant FGF-2 or FGF-2-CaaX were incubated in a reticulocyte lysate system in the presence of [3H]farnesyl pyrophosphate in the absence or presence of B581. Samples were treated with heparin-Sepharose (lanes 1-3) or subjected to immunoprecipitation using anti-FGF-2 antibodies adsorbed to protein A-Sepharose (lanes 4-6) and analyzed by SDS-PAGE and fluorography. The arrow indicates the migration of farnesylated FGF-2-CaaX. (B) Serum-starved NIH/3T3 cells were treated with heparin and the indicated amount of FGF-2 or FGF-2-CaaX for 10 min. The cells were lysed in SDS sample buffer, sonicated, and analyzed by SDS-PAGE and Western blotting using antibodies against total p44/42 MAP kinase (bottom panel). The membrane was stripped and reprobed using antibodies against the phosphorylated form of p44/42 MAP kinase (top panel). (C) Serum-starved NIH/3T3 cells were treated with heparin and increasing amounts of FGF-2 or FGF-2-CaaX for 24 h. During the last 6 h of incubation, 1 μCi/ml [3H]thymidine was present in the medium. The cells were extracted with 5% TCA and the radioactivity incorporated into TCA-insoluble material was measured. (D) FGF-2 or FGF-2-CaaX conjugated to Alexa 488 maleimide was added to NIH/3T3 cells transfected with pcDNA3 vector encoding FGFR1 and incubated for 15 min at 37°C. The cells were then fixed, permeabilized, and treated with a mouse antibody against EEA1. The cells were further treated with Cy3-conjugated anti-mouse antibody. The arrows point to examples of structures positive for EEA1 and FGF-2 or FGF-2-CaaX.
Biological Activity of FGF-2-CaaX. To test the biological activity of FGF-2-CaaX, we first measured the ability of the modified protein to activate p44/42 MAP kinase pathway in NIH/3T3 cells. Cells treated with different amounts of wild-type and mutant growth factor were analyzed by Western blotting using antibodies against the phosphorylated form of p44/42 MAP kinase (Figure 1B, top panel). It is evident that less p44/42 MAP kinase became phosphorylated in cells treated with 1 ng/ml FGF-2-CaaX (lane 4) than when the same amount of unmodified growth factor was used. The mutant growth factor induced, however, full activation of MAP kinase when used at a concentration of 10 ng/ml or higher (lanes 5 and 6). In Figure 1B (bottom panel) antibodies recognizing total p44/42 kinase were used. The results demonstrate that in each case equal amounts of protein were loaded onto the gel.
We also tested the ability of FGF-2-CaaX to stimulate DNA synthesis in NIH/3T3 cells. The data in Figure 1C demonstrate that FGF-2-CaaX was ∼10 times less potent than FGF-2 in stimulating DNA synthesis in serum-starved cells, thus reflecting the impaired ability to activate p44/42 MAP kinase pathway. Nevertheless, at higher concentrations (>10 ng/ml) the mutant growth factor stimulated DNA synthesis to the same level as the unmodified growth factor.
To test if FGF-2-CaaX is endocytosed like FGF-2, they were both labeled with Alexa 488 and added to cells for 15 min at 37°C. The cells were fixed, permeabilized, and stained with antibodies to the early endosome antigen, EEA1. The data in Figure 1D demonstrate that FGF-2 with or without a CaaX-box colocalized with EEA1 indicating that they were present in early endosomes. Some structures stained for FGF-2 or FGF-2-CaaX only and probably represent later compartments in the endocytic pathway.
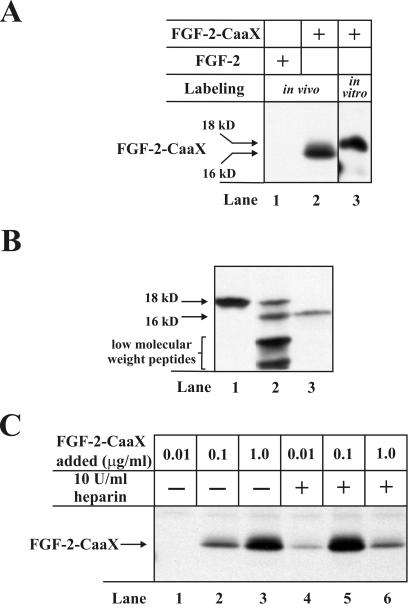
In Vivo Prenylation of FGF-2-CaaX. Next, we investigated whether externally added FGF-2-CaaX is farnesylated by living cells, which would indicate that it is translocated across cellular membranes to reach the cytosol and/or the nucleus. NIH/3T3 cells were starved for serum, then incubated with radioactive mevalonate in the presence of lovastatin to reduce endogenous synthesis of mevalonate, and finally treated with heparin and FGF-2 or FGF-2-CaaX for 6 h. The cells were then lysed, and treated with heparin-Sepharose, and the adsorbed material was analyzed by SDS-PAGE. Figure 2A demonstrates that in cells treated with FGF-2-CaaX (lane 2), but not in cells treated with FGF-2 (lane 1) there was a labeled band with migration rate corresponding to ∼16 kDa. This is less than the expected 18 kDa (lane 3), presumably as a result of proteolytical processing of the 18-kDa isoform (see below). Because the farnesylation signal is located at the C-terminus, the cleavage must take place from the N-terminal end.
Figure 2.
In vivo prenylation of FGF-2-CaaX in NIH/3T3 cells. (A) After 24 h serum-starved cells were preincubated for 2 h with [14C]mevalonolactone and 1 μg/ml lovastatin and then treated for 6 h with heparin and either FGF-2 (lane 1) or FGF-2-CaaX (lane 2). The cells were lysed and the cellular material was adsorbed onto heparin-Sepharose and analyzed by SDS-PAGE and fluorography. The arrows indicate the migration of in vivo prenylated and in vitro farnesylated FGF-2-CaaX. In vitro farnesylated FGF-2-CaaX (lane 3) is shown as marker. (B) Serum-starved cells were incubated for 6 h with heparin and ∼10 ng/ml [35S]methionine-labeled 18-kDa FGF-2. Cells were washed with 2 M NaCl in Na-acetate buffer, pH 4.0, and lysed. The material present in the high-salt/low-pH wash (lane 1), and the cellular material (lane 2) was adsorbed onto heparin-Sepharose and analyzed by SDS-PAGE and fluorography. [35S]methionine-labeled 16-kDa form of FGF-2 (lane 3) is shown as marker. (C) Cells were pretreated as in A and then incubated for 6 h with the indicated amounts of FGF-2-CaaX in the absence (lanes 1-3) or presence (lanes 4-6) of heparin. After lysis, cellular material was analyzed as in A.
Control experiments showed that there was no labeling of FGF-2-CaaX when the protein was added after cell lysis (not demonstrated), indicating that prenylation of the growth factor occurred only in the intact and living cells. The data therefore indicate that FGF-2-CaaX is translocated into living cells.
Intracellular Processing of FGF-2 upon Entry into Cells.
The in vivo prenylation experiments above suggested that concomitantly with translocation the FGF-2-CaaX was cleaved at the N-terminus, which is susceptible to proteolytical degradation (Gospodarowicz, 1987; Doble et al., 1990; Sperinde and Nugent, 2000). To further test this we synthesized the 18-kDa form of FGF-2 in a rabbit reticulocyte lysate in the presence of [35S]methionine. The labeled growth factor was added to cells in the presence of heparin and incubated for 6 h. The cells were washed with 2 M NaCl in Na-acetate buffer, pH 4.0, which removes the growth factor bound to cell surface, and then lysed. The cellular material and the material present in the high-salt/low-pH wash were adsorbed onto heparin-Sepharose and analyzed by SDS-PAGE and fluorography.
The data in Figure 2B show that the growth factor that could be removed from the cell surface by a high-salt/low-pH wash was exclusively in the 18-kDa form (lane 1). In contrast, the internalized growth factor was partially processed to yield a short form of ∼16 kDa and further degraded into low-molecular-weight peptides of <10 kDa (lane 2). Lane 3 demonstrates the migration of the in vitro synthesized 16-kDa form for comparison. The data indicate that the cleavage of 18-kDa FGF-2 does not take place at the cell surface but occurs after endocytosis of the growth factor.
Effect of Heparin on Growth Factor Translocation. We then tested the concentration dependence of FGF-2-CaaX translocation in NIH/3T3 cells in the absence and presence of heparin. The data in Figure 2C show that in the absence of heparin small amounts of labeled FGF-2-CaaX could be detected when cells were treated with 100 ng/ml protein (lane 2), and a much stronger labeling was observed when cells were treated with 1 μg/ml growth factor (lane 3). On the other hand, in the presence of heparin the prenylated FGF-2-CaaX could be detected already when cells were treated with 10 ng/ml protein (lane 4). In the presence of heparin an optimal level of FGF-2-CaaX labeling was found when cells were treated with ∼100 ng/ml protein (lane 5), whereas further increase in the growth factor concentration (up to 1 μg/ml) resulted in less labeling (lane 6). In our further experiments we therefore used 10 U/ml heparin and 100 ng/ml modified growth factor.
Comparison with Translocation of FGF-1
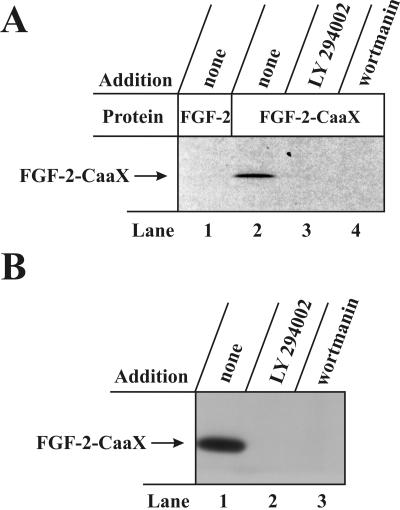
PI3 kinase Is Required for FGF-2 Translocation. It was previously shown by Klingenberg et al. (2000) that translocation of external FGF-1 to cytosol and/or nucleus requires the activity of PI3 kinase. Thus, inhibitors of PI3 kinase such as LY294002 and wortmannin blocked translocation. We tested whether FGF-2 translocation would also require the activity of PI3 kinase. Figure 3A shows that prenylation of FGF-2-CaaX in CPAE cells was completely blocked in the presence of LY294002 (lane 3) and wortmannin (lane 4). Similar experiments were performed in NIH/3T3 cells (Figure 3B), and also in this case prenylation of FGF-2-CaaX was completely blocked by LY294002 (lane 2) and wortmannin (lane 3). This indicates that translocation of FGF-2 requires PI3 kinase activity.
Figure 3.
Effect of inhibitors of PI-3 kinase on the prenylation of FGF-2-CaaX in vivo. (A) CPAE cells were pretreated as in Figure 2A and then treated for 6 h with heparin and either FGF-2 (lane 1) or FGF-2-CaaX (lanes 2-4). The incubation was carried out in the absence (lanes 1 and 2) or presence of 50 μM LY294002 (lane 3), or 200 nM wortmannin (lane 4). After lysis, cellular material was analyzed as in Figure 2A. (B) NIH/3T3 cells were pretreated as in Figure 2A and then treated for 6 h with heparin and FGF-2-CaaX. The incubation was carried out in the absence (lane 1) or presence of 50 μM LY294002 (lane 2) or 150 nM wortmannin (lane 3). After lysis, cellular material was analyzed as in Figure 2A.
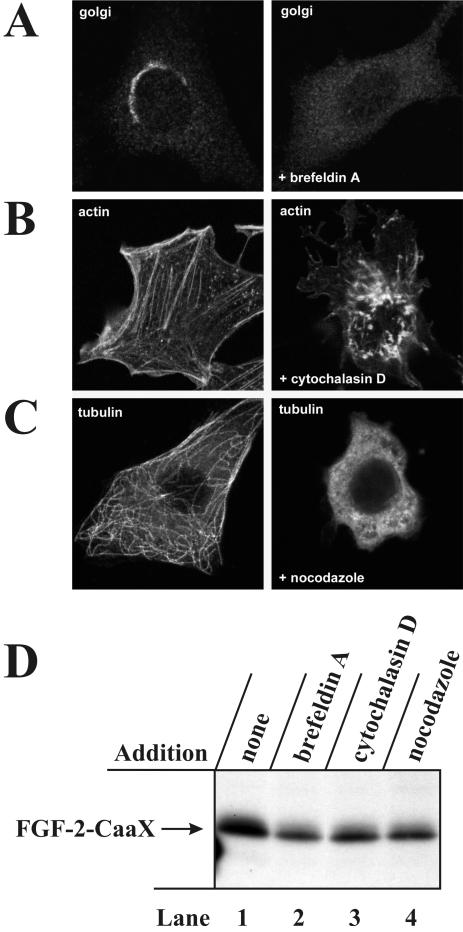
Translocation of FGF-2 Is Insensitive to Treatment with Brefeldin A, Nocodazole, and Cytochalasin D. We then tested whether translocation of FGF-2-CaaX involves the Golgi apparatus. In NIH/3T3 cells treated with 2 μg/ml brefeldin A, which induces disruption of the Golgi apparatus (Figure 4A), the farnesylation of FGF-2-CaaX was not blocked (Figure 4D, lane 2), although there was some reduction (∼40%) in the amount of labeled growth factor compared with the control (Figure 4D, lane 1). This could be due to a toxic effect on the cells exerted by the long-term treatment (6 h) with the drug.
Figure 4.
In vivo prenylation of FGF-2-CaaX is not blocked by treatment with brefeldin A, nocodazole and cytochalasin D. (A) NIH/3T3 cells were treated without or with 2 μg/ml brefeldin A for 30 min. The Golgi apparatus was visualized with anti-β-COP and Cy3-conjugated anti-rabbit antibody using confocal microscopy. (B) NIH/3T3 cells were treated without or with 10 μg/ml cytochalasin D for 30 min. Actin was visualized with rhodamine-conjugated phalloidin. (C) NIH/3T3 cells were treated without or with 33 μM nocodazole for 30 min. Microtubuli were visualized with antitubulin and rhodamine-conjugated anti-mouse antibody. (D) NIH/3T3 cells were pretreated as in Figure 2A and then treated for 6 h with heparin and FGF-2-CaaX in the absence (lane 1) or presence of 2 μg/ml brefeldin A (lane 2), 10 μg/ml cytochalasin D (lane 3), or 33 μM nocodazole (lane 4). After lysis, cellular material was analyzed as in Figure 2A.
Also treatment of NIH/3T3 cells with cytochalasin D (Figure 4D, lane 3), which disrupts actin microfilaments (Figure 4B) or with nocodazole (Figure 4D, lane 4), which disrupts microtubules (Figure 4C), did not influence the farnesylation of FGF-2-CaaX to a large extent. It therefore appears that, as in case of FGF-1 (Malecki et al., 2002), the Golgi apparatus is not the site of FGF-2 translocation and that retrograde transport across the Golgi is also not required. In addition, intact microtubule and microfilament networks do not appear to be required for translocation of either growth factor.
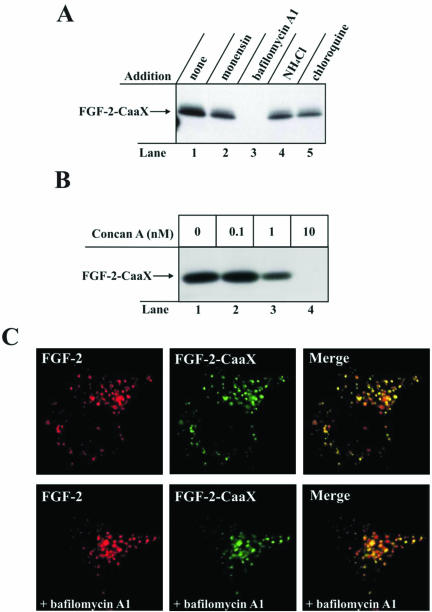
Translocation of FGF-2 Requires Vesicular Membrane Potential. We have earlier provided evidence that translocation of FGF-1 requires a membrane potential (positive inside) across the limiting membrane of intracellular vesicles containing the vesicular type proton pumps (Malecki et al., 2002). We therefore tested if translocation of FGF-2 had similar requirements. Figure 5A shows, that in NIH/3T3 cells prenylation of FGF-2-CaaX in the presence of monensin (lane 2), NH4Cl (lane 4), or chloroquine (lane 5) was similar to the control (lane 1). However in the presence of bafilomycin A1, which inhibits the vesicular type proton pumps, the translocation of the growth factor was completely blocked (lane 3). Also concanamycin A, another inhibitor of the proton pumps, fully blocked prenylation of FGF-2-CaaX (Figure 5B) when used at a concentration of 10 nM (lane 4).
Figure 5.
Effect of various inhibitors of lumenal acidification of intracellular vesicles on the ability of FGF-2-CaaX to become prenylated in vivo. (A) NIH/3T3 cells were pretreated as in Figure 2A and then treated for 6 h with heparin and FGF-2-CaaX in the absence (lane 1) or presence of 1 μM monensin (lane 2), 10 nM bafilomycin A1 (lane 3), 20 mM NH4Cl (lane 4), or 100 μM chloroquine (lane 5). After lysis, cellular material was analyzed as in Figure 2A. (B) NIH/3T3 cells were pretreated as in Figure 2A and then treated for 6 h with heparin and FGF-2-CaaX in the absence (lane 1) or the presence of increasing concentrations of concanamycin A (lanes 2-4). After lysis, cellular material was analyzed as in Figure 2A. (C) FGF-2 conjugated to Cy3 maleimide and FGF-2-CaaX conjugated to Alexa 488 maleimide were added to NIH/3T3 cells overexpressing FGFR1 and incubated for 2 h at 37°C. In some cases, the cells were pretreated with 10 nM bafilomycin A. The cells were then fixed and analyzed by confocal microscopy.
To test if bafilomycin A1 interfered with the trafficking of FGF-2 or FGF-2-CaaX or if the introduced CaaX-box some-how altered the transport of the growth factor, we labeled FGF-2 with Cy3 and FGF-2-CaaX with Alexa 488. The labeled growth factors were both added to cells and analyzed by confocal microscopy. The data in Figure 5C demonstrate that after 2-h incubation at 37°C, FGF-2 and FGF-2-CaaX were present in the same endosomal compartments and that there was no apparent difference whether or not bafilomycin A1 was present.
At the concentrations used above, all tested compounds inhibit acidification of internal vesicles or otherwise increase their lumenal pH. Treatment with bafilomycin A1 or concanamycin A, which are selective inhibitors of V-type H+-ATPase (Drose and Altendorf, 1997), results also in dissipation of the vesicle transmembrane potential, which is not the case with monensin, NH4Cl, or chloroquine. Therefore, the results in Figure 5A and 5B indicate that translocation of FGF-2 to the cytosol requires active vesicular proton pumps and that the electrical potential across the vesicle membrane (positive inside), but not the pH gradient (acidic inside), is required.
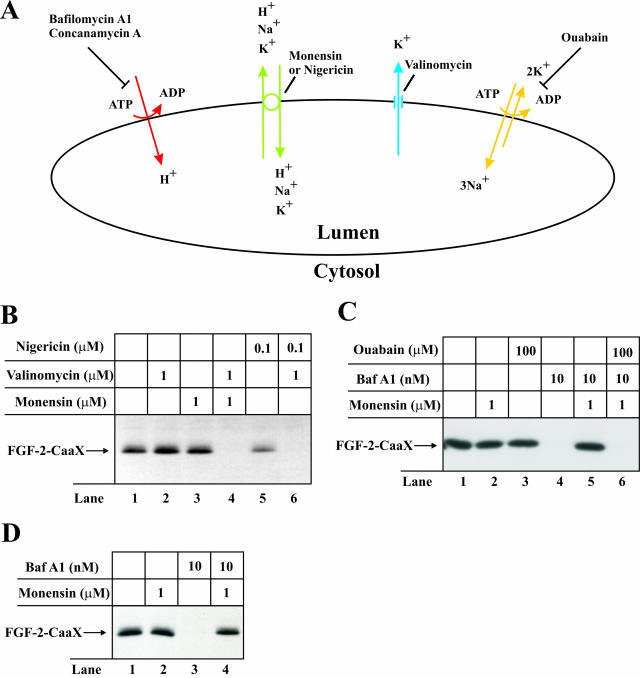
To test this possibility, we carried out the prenylation assay in cells treated with combinations of ionophores to depolarize the vesicular membrane. Monensin and nigericin are both electroneutral monovalent cation exchangers, whereas valinomycin is an electrogenic K+ ionophore that transports K+ in accordance with existing chemical and electrical gradients. In the presence of active proton pumps, treatment with monensin (or nigericin) alone will induce intravesicular accumulation of K+ ions as a result of exchange with lumenal H+. This will lead to neutralization of the vesicular pH without change in the vesicular membrane potential. Under such conditions, addition of valinomycin will cause leakage of accumulated K+ ions from the vesicle to the cytosol and, as consequence, depolarization of the vesicular membrane (Figure 6A).
Figure 6.
Effect of depolarization and repolarization of the membrane of intracellular vesicles on the ability of FGF-2-CaaX to become prenylated in vivo. (A) H+ are pumped from the cytosol into the lumen of the vesicles by V-type H+-ATPase (red) lowering lumenal pH and creating a membrane potential (positive inside the vesicle). The membrane potential is partly compensated for by the influx of Cl- counterions (not indicated). Inhibition of vacuolar proton pumps by bafilomycin A1 or concanamycin A causes depolarization of the membrane. Monensin or nigericin (green) exchange H+ accumulated in the vesicle lumen for K+ present in the cytosol, which raises the lumenal pH but does not dissipate the membrane potential. When both monensin (or nigericin) and valinomycin (blue) are present, efflux of K+ from the lumen to the cytosol results in the dissipation of the membrane potential. Endosomes also contain Na+/K+-ATPase (yellow), which is usually inactive because of deficiency in lumenal K+. In the presence of monensin (or nigericin) lumenal K+ can be replenished (in exchange for Na+) leading to reactivation of the Na+/K+-ATPase and regeneration of the membrane potential even when V-type H+-ATPase is blocked. Under these conditions, treatment with ouabain (inhibitor of Na+/K+-ATPase) will lead again to depolarization of the membrane. (B) CPAE cells were pretreated as in Figure 2A and then treated for 6 h with heparin and FGF-2-CaaX in the absence (lane 1) or presence of valinomycin (lane 2), monensin (lane 3), nigericin (lane 5), or a combination of valinomycin and either monensin (lane 4) or nigericin (lane 6). After lysis, cellular material was analyzed as in Figure 2A. (C) NIH/3T3 cells were pretreated as in Figure 2A and then treated for 6 h with heparin and FGF-2-CaaX in the absence (lane 1) or presence of monensin (lane 2), ouabain (lane 3), bafilomycin A1 (lane 4), or a combination of both bafilomycin A1 and either monensin (lane 5) or monensin and ouabain (lane 6). After lysis, cellular material was analyzed as in Figure 2A. (D) CPAE cells were pretreated as in Figure 2A and then treated for 6 h with heparin and FGF-2-CaaX in the absence (lane 1) or presence of monensin (lane 2), bafilomycin A1 (lane 3), or a combination of both monensin and bafilomycin A1 (lane 4). After lysis, cellular material was analyzed as in Figure 2A.
The results in Figure 6B show that in CPAE cells treated with valinomycin alone (lane 2) prenylation of FGF-2-CaaX was similar as in control cells (lane 1), indicating that [K+] in the vesicles was not higher than in the cytosol. Translocation of FGF-2-CaaX in cells treated with monensin alone (lane 3) or nigericin alone (lane 5) was also similar as in control cells. On the other hand, treatment with the combination of valinomycin and monensin (lane 4) or valinomycin and nigericin (lane 6) resulted in a complete block of FGF-2-CaaX labeling. Similar results were obtained when the prenylation assay was carried out in NIH/3T3 cells (our unpublished results). The data indicate that depolarization of the vesicular membrane by combination of the two ionophores blocks the translocation of FGF-2-CaaX.
Recovery of Translocation by Restoration of the Vesicular Membrane Potential. Next, we tested if regeneration of the vesicular membrane potential would allow translocation of FGF-2 in the absence of active vacuolar H+ pumps. It has been reported that endocytic vesicles contain Na+/K+-ATPase (Cain et al., 1989; Fuchs et al., 1989). In the absence of active K+ channels the Na+/K+-ATPase will soon be inactive because of limiting concentration of vesicular K+. We reasoned that treatment with monensin could reactivate the Na+/K+-ATPase by providing the necessary K+. In this case, even in the absence of active vesicular proton pumps (e.g., blocked by bafilomycin A1) an inside-positive vesicle membrane potential could build up and support translocation of FGF-2. Treatment with ouabain, an inhibitor of the Na+/K+-ATPase, should block translocation under these conditions (Figure 6A).
The data presented in Figure 6C are in favor of the above model. Treatment of NIH/3T3 cells with bafilomycin A1 prevented translocation of FGF-2 (lane 4). However when bafilomycin A1 was used in combination with monensin (lane 5), the translocation was restored. On the other hand, upon simultaneous addition of ouabain (lane 6) the translocation was blocked again. There was no effect of ouabain alone (lane 3). FGF-2 translocation blocked by treatment with bafilomycin A1 could also be restored by monensin in CPAE cells (Figure 6D). Therefore, restoration of the vesicle membrane potential allows the translocation of FGF-2 to reoccur.
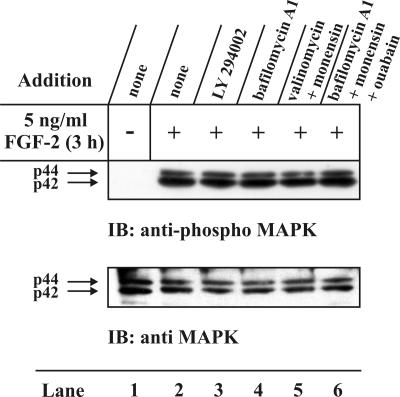
Tested Compounds Do Not Affect FGF-2 Binding or FGF-receptor Activation. Interaction of FGF-2 with specific FGF receptors is considered to trigger the response of target cells to the growth factor treatment. Therefore, any change in the binding of FGF-2 to specific FGF receptors is likely to influence the overall cellular response. We therefore considered the possibility that inhibition of FGF-2 translocation upon drug treatment could be due to interference with binding of the growth factor to its receptor or to interference with receptor activation. It was shown previously (Klingenberg et al., 2000; Malecki et al., 2002) that LY294002, wortmannin, bafilomycin A1, or combination of monensin and bafilomycin A1 or combination of monensin and valinomycin had little or no effect on the apparent affinity and the number of binding sites for FGF-1 at the surface of NIH/3T3 cells.
We here tested the extent of MAP kinase phosphorylation in NIH/3T3 cells, which is indicative of FGF receptor activation. The results in Figure 7 (top panel) show that after 24 h serum-starvation the MAP kinase p44/42 was mostly in the nonphosphorylated form (lane 1). On treatment with heparin and FGF-2 for 3 h there was a dramatic increase in the amount of phosphorylated MAP kinase (lane 2). The phosphorylation of p44/42 MAP kinase was not affected by pretreatment with LY294002 (lane 3), bafilomycin A1 (lane 4), by the combination of monensin and valinomycin (lane 5), or by the combination of monensin, bafilomycin A1, and ouabain (lane 6). Figure 7 (bottom panel) shows that in each case similar amounts of protein were loaded onto the gel.
Figure 7.
Effect of various inhibitors and their combinations on the ability of FGF-2 to stimulate phosphorylation of MAP-kinase in NIH/3T3 cells. Serum-starved cells were preincubated for 2 h in the absence (lanes 1 and 2) or presence of 50 μM LY294002 (lane 3), 10 nM bafilomycin A1 (lane 4), or a combination of 1 μM monensin and either 1 μM valinomycin (lane 5) or 10 nM bafilomycin A1 and 100 μM ouabain (lane 6). The cells were left untreated (lane 1) or treated for 3 h with heparin and 5 ng/ml FGF-2 (lanes 2-6). The cells were subsequently lysed and analyzed by Western blotting with anti-p44/42 MAP-kinase antibodies (bottom panel). The membrane was stripped and reprobed with antiphosphorylated-p44/42 MAP-kinase antibodies (top panel).
Translocation of FGF-1 and FGF-2 Occurs during G1
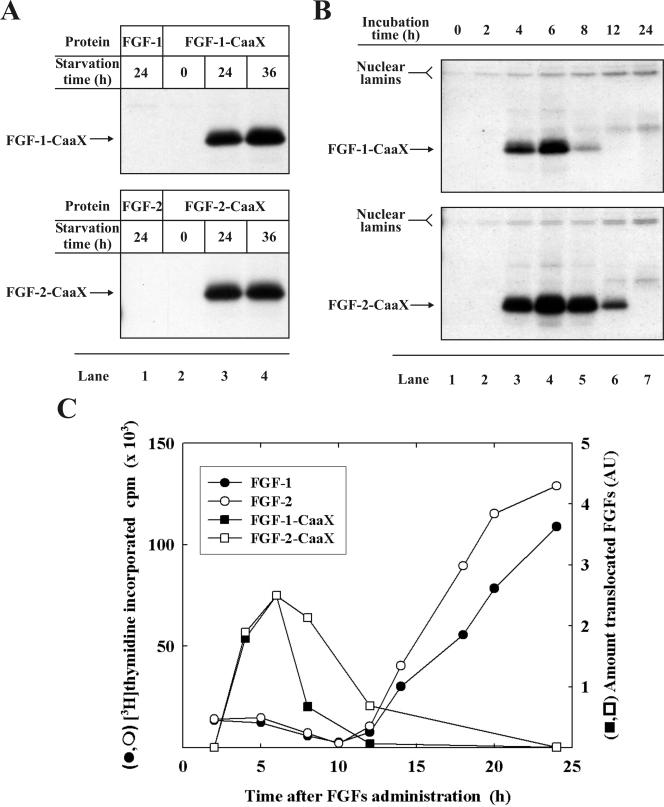
Cell Cycle Dependence of FGF-1 and FGF-2 Translocation. In our present as well as earlier experiments (Malecki et al., 2002) we used serum-starved cells. Figure 8A shows the results of in vivo prenylation of FGF-1-CaaX (top panel) and FGF-2-CaaX (bottom panel) in NIH/3T3 cells that were serum-starved for different periods of time. It is evident that in cells which were not serum-starved, prenylation of FGF-2-CaaX was below the detection level (lane 2). On the other hand there was little difference in the amount of labeled growth factors between cells that were serum-starved for 24 h (lane 3) or 36 h (lane 4).
Figure 8.
Dependence of FGF-1 and FGF-2 translocation on the cell cycle in NIH/3T3 cells. (A) Cells were serum-starved for the indicated period of time and then preincubated for 2 h with [14C]mevalonolactone and 1 μg/ml lovastatin. Next, the cells were treated for 6 h with heparin and either: FGF-1 and FGF-1-CaaX (top panel), or FGF-2 and FGF-2-CaaX (bottom panel). After lysis, cellular material was analyzed as in Figure 2A. (B) Cells were pretreated as in Figure 2A. Next, the cells were treated for the indicated period of time with heparin and either: FGF-1-CaaX (top panel) or FGF-2-CaaX (bottom panel). After lysis, cellular material was analyzed as in Figure 2A. (C) Cells were serum-starved for 24 h and treated with heparin and 10 ng/ml either: FGF-1 (•) or FGF-2 (○) for the indicated period of time. During the last 1 h of incubation 1 μCi/ml [3H]thymidine was present in the medium. Next, cells were precipitated with 5% TCA and the incorporated radioactivity was measured. For comparison the same graph shows the plot of band intensities from B (in arbitrary units), which represent prenylated FGF-1-CaaX (▪) or prenylated FGF-2-CaaX (□).
The requirement of serum starvation for efficient translocation of the growth factors suggests a dependence on the cell cycle. Because serum-starved cells arrest mainly in early G1, this phase was therefore an obvious candidate.
Figure 8B shows the results of a time-course experiment of FGF-1-CaaX (top panel) and FGF-2-CaaX (bottom panel) translocation in serum-starved cells. There was hardly any prenylation of the growth factors within the first 2 h of incubation (lane 2). When longer periods of incubation were used, the amount of labeled material gradually increased to reach an apparent maximum after ∼6 h (lane 4). Further incubation resulted in a decrease of the labeling of the growth factors. After 24-h incubation the amount of translocated FGF-1 and FGF-2 was undetectable (lane 7), although prenylation of endogenous nuclear lamins (which adsorb to heparin-Sepharose to some extent) was further increased.
To determine the relative time-positioning of translocation and S-phase, serum-starved cells were treated with FGF-1 or FGF-2 for increasing periods of time, and the incorporation of [3H]thymidine was measured during the last 1 h. The results presented in Figure 8C show that DNA synthesis starts ∼12 h after treatment with the growth factors and continues for several hours (>24 h). Therefore, the translocation of FGF-1 and FGF-2 occurs during most of the G1 phase and the amount of translocated growth factors decreases shortly before the cells enter into S-phase (before the G1→S transition).
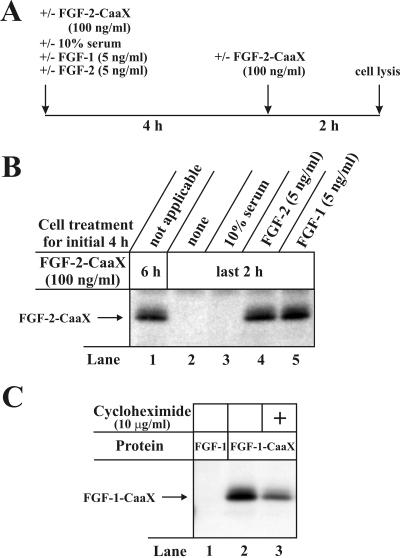
Translocation of FGF-2 Can Be Induced by both FGF-1 and FGF-2 Treatment. We were intrigued by the occurrence of an apparent lag-phase (>2 h) between the treatment of cells with the growth factor and the onset of translocation (Figure 8B). We suspected that this lag-phase was necessary for switching on the translocation machinery and we questioned whether this could be achieved by alternative treatments of the cells. Figure 9B, lane 1, shows the prenylation of FGF-2-CaaX after 6 h of continuous incubation with the growth factor. When cells were initially rested for 4 h and then treated with FGF-2-CaaX during the last 2 h of incubation, the growth factor was not labeled (lane 2). When cells were preincubated with 10% serum for 4 h and then treated with FGF-2-CaaX for 2 h, the growth factor was also not labeled (lane 3). Labeling occurred, however, in cells preincubated for 4 h in the presence of 5 ng/ml FGF-2 (lane 4). Even though FGF-2-CaaX was added only for the last 2 h of incubation the labeling was similar as in the case when CaaX-containing growth factor was present for the entire 6 h. Interestingly, preincubation of cells for 4 h with 5 ng/ml FGF-1 (lane 5) also resulted in translocation of FGF-2-CaaX within 2 h. The results indicate that the cellular machinery used for translocation of FGF-2 is induced in cells by treatment with either FGF-1 or FGF-2 but not by treatment with serum.
Figure 9.
FGF-2 translocation is induced by FGF-1 and FGF-2 treatment of NIH/3T3 cells. (A) Schematic representation of the time-course of the experiment. (B) Serum starved cells were preincubated with [14C]mevalonolactone, 1 μg/ml lovastatin and heparin. In one case 100 ng/ml FGF-2-CaaX was added for the entire 6 h incubation (lane 1). In other cases, the cells were for the initial 4 h left untreated (lane 2) or were treated with 10% serum (lane 3), 5 ng/ml FGF-2 (lane 4) or 5 ng/ml FGF-1 (lane 5) and then 100 ng/ml FGF-2-CaaX was added for the final 2 h of incubation. After lysis, cellular material was analyzed as in Figure 2A. (C) NIH/3T3 cells were pretreated as in Figure 2A and then treated for 6 h with heparin and either FGF-1 or FGF-1-CaaX in the absence (lanes 1 and 2) or presence of cycloheximide (lane 3). After lysis, cellular material was analyzed as in Figure 2A.
Cycloheximide Does Not Block Translocation. Next, we tested whether translocation of the growth factor required synthesis of new proteins. Figure 9C shows that treatment of cells with FGF-1-CaaX in the presence of cycloheximide (lane 3) did not prevent labeling of the growth factor, although the efficiency was reduced (∼40%) compared with the control (lane 2). Parallel experiments confirmed that in cells treated with 10 μg/ml cycloheximide the protein synthesis was completely blocked (not demonstrated). The results indicate that translocation is not dependent on de novo protein synthesis.
DISCUSSION
The main findings in the present article is that externally added FGF-2, which is modified to contain a farnesylation signal, is translocated to the cytosol by a mechanism that requires active PI3 kinase and an electrical potential across the limiting membrane of intracellular vesicles containing the vesicular type proton pumps. This strongly indicates that the translocation occurs from an intracellular vesicular compartment. Concomitantly with the translocation FGF-2 is cleaved at the N-terminus, which results in an increase in migration rate in SDS-PAGE from 18 to 16 kDa apparent molecular weight. Translocation of both FGF-1 and FGF-2 seems to occur during most of the G1-phase. The intracellular concentration of exogenous growth factors reaches an apparent maximum ∼6 h after beginning of growth factor treatment and decreases shortly before the cells enter into S-phase.
Because it has been reported that treatment of cells with FGF-2 may induce expression of FGF-2 in the target cells (Peng et al., 2001; Cowan et al., 2003), it is essential to be able to differentiate between the growth factor that is added to the cells and growth factor produced by the cells. We here document that FGF-2 that has not been modified to contain a CaaX-box is not labeled when the cells are incubated with radioactive mevalonate, a precursor of the farnesyl group.
The farnesylation signal consists of four amino acids added to the C-terminal end of the protein. It is unlikely that this will strongly affect the translocation of the growth factor and it is therefore likely that the conclusions made for FGF-2-CaaX can be extended to the unmodified growth factor. This appears to be the case with the related FGF-1 (Wiȩdlocha et al., 1995) as well as with diphtheria toxin (Falnes et al., 1995).
It should be noted, however, that at low concentrations of the growth factor the biological activity of FGF-2-CaaX was somewhat lower that that of the unmodified FGF-2, both in its ability to induce phosphorylation of p44/42 MAP kinase and to stimulate DNA synthesis. The most likely explanation is that addition of the CaaX-box impairs the ability of the growth factor to bind to and/or activate high-affinity FGF receptors. Nevertheless, in the concentration regime of growth factors used throughout this study (∼100 ng/ml), both FGF-2 and FGF-2-CaaX exerted similar biological effects.
Vesicular type proton pumps are present in endosomes, lysosomes and in the Golgi apparatus. Because brefeldin A treatment did not block the translocation, it is unlikely that the translocation occurs from the Golgi apparatus and/or that the retrograde transport across the Golgi to the ER is required. Translocation was not blocked by nocodazole, which disrupts microtubule network required for vesicles that traffic along the degradative pathway (Bomsel et al., 1990). This makes the lysosomes an unlikely location for translocation. Therefore, endosomes appear as the most likely translocation site for FGF-2 similarly to FGF-1, for which we earlier found that it accumulates in the juxtanuclear recycling endosomal compartment (Citores et al., 1999). The data here presented indicate that the endosomes where the translocation takes place possess not only vesicular-type proton pumps, but also Na+/K+-ATPase and can be characterized by the lack of active K+ channels.
Treatment of the cells with brefeldin A, nocodazole, and cytochalasin D did not block the translocation measured as farnesylation of the growth factor. On the other hand, there was in each case a certain reduction in labeling in the presence of these drugs. In the case of nocodazole and cytochalasin D we believe this is due to toxic effects of the drugs in the rather long incubation period (6 h) during which the cells clearly change morphology. In the case of brefeldin A, the reduced labeling would be expected due to the fact that new FGF-receptors are unable to reach the cell surface and therefore the binding capacity of the cells for FGF-2 is probably reduced.
The data here presented strongly indicate that electric potential across the limiting membrane of intracellular vesicles is essential for translocation to occur. The reason for this is so far not clear. The transmembrane potential could have a direct role as a driving force for translocation. It could also be involved indirectly, e.g., by affecting the structure of transmembrane proteins involved in the translocation process or by impairing the vesicular transport to the site of translocation.
The biological role of the intracellular translocated FGF-2 is unclear. In case of FGF-1 it was shown that translocated growth factor can trigger the mitogenic response, at least in certain cell types (Wiȩdlocha et al., 1996). Interestingly, the mitogenic activity of FGF-1 variants correlates with their ability to be phosphorylated by protein kinase C (Klingenberg et al., 1998) and with their ability to interact with protein kinase CK2 (Skjerpen et al., 2002)—a ubiquitous serine/threonine protein kinase necessary for cell cycle progression (Pepperkok et al., 1994). Also FGF-2 wild-type, but not a nonmitogenic variant, was reported to interact with protein kinase CK2 (Bailly et al., 2000). The observation that translocation of both FGF-1 and FGF-2 takes place during G1 suggests that these growth factors may indeed play some direct role in the entry and progress through early stages of the cell cycle. Intriguing in this respect is the reported observation that FGF-2 is able to stimulate transcription in cell-free systems (Bouche et al., 1987; Nakanishi et al., 1992).
We found that the intracellular concentration of translocated FGF-1 and FGF-2 decreased shortly before the start of DNA synthesis. Because prenylation of control proteins (e.g., nuclear lamins) was increased throughout the incubation, it is unlikely that the decrease in the labeling of FGFs-CaaX is due to simple removal of the prenyl group. It can be argued, however, that the mutation decreases the intracellular life span of the CaaX-containing growth factors. Although this cannot be excluded, an earlier report with125IFGF-1 demonstrated that nuclear-associated growth factor becomes undetectable after prolonged (14 h) incubation with BALBc/3T3 cells (Zhan et al., 1993). It is therefore most likely that the decrease in translocated FGF-1 and FGF-2 before the onset of S-phase is due to combined inhibition of translocation of new growth factor and degradation of the already translocated growth factors, which may become dispensable at this point of the cell cycle.
On endocytosis of the growth factor, some part of the 18-kDa FGF-2 is processed to yield a short form of ∼16 kDa. Because the FGF-2-CaaX prenylated in vivo is present exclusively in the 16-kDa form and the farnesylation signal is located at the C-terminus, the cleavage must take place from the N-terminal end. The effect of the cleavage on the function of the growth factor is not clear. The 18- and 16-kDa forms isolated from tissues appear to stimulate FGF receptors to the same extent (Gospodarowicz, 1987). It is possible, however, that the two forms differ in their intracellular activity. The 18-kDa FGF-2 is synthesized in the cytosol and posttranslationally exported by a poorly defined mechanism. Its subsequent internalization and cleavage to the 16-kDa form could result in the appearance in the cytosol of a growth factor with properties different from those of the 18-kDa form. Further work will be required to elucidate this question.
Most of our prenylation experiments were done in the presence of heparin. Although we observed labeling of FGF-2-CaaX also in the absence of heparin, considerably more protein had to be used to obtain efficient translocation. This is in accordance with the finding that the binding of the growth factor to specific FGF-receptors is increased in the presence of heparin (Ornitz and Leder, 1992). In addition, the presented results indicate that in the presence of heparin, translocation of FGF-2-CaaX is optimal at concentration of ∼100 ng/ml protein, whereas at higher concentrations the amount of translocated growth factor is less. This is in accordance with the work of Spivak-Kroizman et al. (1994), who showed that at elevated concentrations of FGF-1, the dimerization of FGF receptors is much reduced. Most likely the same mechanism applies to other FGFs, which would again indicate that translocation of FGF-2 is dependent on FGF receptors.
Another interesting observation is the occurrence of a certain lag period between initial growth factor treatment and the onset of translocation. Because translocation of FGF-1 was not blocked in cells treated with cycloheximide, the lag period does not result from de novo protein synthesis but rather from the activation and/or assembly of preexisting components of the translocation machinery. The “switching-on” of the translocation machinery can be achieved by treatment with both FGF-1 and FGF-2, which reinforces the general conclusion that both growth factors use a common mechanism for translocation. It should be noted that a certain reduction in the prenylation of the growth factor is rather expected during prolonged (6 h) incubations of cells with cycloheximide. Inhibition of protein synthesis will decrease the availability of all proteins involved in translocation (e.g., FGF receptors).
Although similar, FGF-2 clearly differs from FGF-1. The here presented finding that the two growth factors appear to translocate into cells by using a common mechanism (Malecki et al., 2002; present article) opens for the possibility that also other growth factors and cytokines may be translocated using a similar pathway to carry out part of their activity in the cytosol or nucleus (Olsnes et al., 2003).
Acknowledgments
The skilful technical assistance with the cell cultures of Anne Gro Bredesen and Brit Solvår Morken is gratefully acknowledged. C.S.S. is a predoctoral fellow of the Norwegian Cancer Society. J.M. and J.W. are postdoctoral Fellows of The Research Council of Norway and The Norwegian Cancer Society, respectively. This work was supported by Novo Nordisk Foundation, Blix Fund for the Promotion of Medical Research, Rachel and Otto Kr. Bruun's Fund, Torsteds legat, and by The Jahre Foundation.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0589. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0589.
Abbreviations used: FGF, fibroblast growth factor; MAP-kinase, mitogen-activated protein kinase; TCA, trichloroacetic acid.
References
- Arnaud, E., Touriol, C., Boutonnet, C., Gensac, M.C., Vagner, S., Prats, H., and Prats, A.C. (1999). A new 34-kilodalton isoform of human fibroblast growth factor 2 is cap dependently synthesized by using a non-AUG start codon and behaves as a survival factor. Mol. Cell. Biol. 19, 505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly, K., Soulet, F., Leroy, D., Amalric, F., and Bouche, G. (2000). Uncoupling of cell proliferation and differentiation activities of basic fibroblast growth factor. FASEB J. 14, 333-344. [PubMed] [Google Scholar]
- Baldin, V., Roman, A.M., Bosc-Bierne, I., Amalric, F., and Bouche, G. (1990). Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 9, 1511-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel, M., Parton, R., Kuznetsov, S.A., Schroer, T.A., and Gruenberg, J. (1990). Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell 62, 719-731. [DOI] [PubMed] [Google Scholar]
- Bonnet, H., Filhol, O., Truchet, I., Brethenou, P., Cochet, C., Amalric, F., and Bouche, G. (1996). Fibroblast growth factor-2 binds to the regulatory beta subunit of CK2 and directly stimulates CK2 activity toward nucleolin. J. Biol. Chem. 271, 24781-24787. [DOI] [PubMed] [Google Scholar]
- Bossard, C., Laurell, H., Van den Berghe, L., Meunier, S., Zanibellato, C., and Prats, H. (2003). Translokin is an intracellular mediator of FGF-2 trafficking. Nat. Cell Biol. 5, 433-439. [DOI] [PubMed] [Google Scholar]
- Bouche, G., Gas, N., Prats, H., Baldin, V., Tauber, J.P., Teissie, J., and Amalric, F. (1987). Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0-G1 transition. Proc. Natl. Acad. Sci. USA 84, 6770-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain, C.C., Sipe, D.M., and Murphy, R.F. (1989). Regulation of endocytic pH by the Na+, K+-ATPase in living cells. Proc. Natl. Acad. Sci. USA 86, 544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citores, L., Wesche, J., Kolpakova, E., and Olsnes, S. (1999). Uptake and intracellular transport of acidic fibroblast growth factor: evidence for free and cytoskeleton-anchored fibroblast growth factor receptors. Mol. Biol. Cell 10, 3835-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, C.M., Quarto, N., Warren, S.M., Salim, A., and Longaker, M.T. (2003). Age-related changes in the biomolecular mechanisms of calvarial osteoblast biology affect fibroblast growth factor-2 signaling and osteogenesis. J. Biol. Chem. 278, 32005-32013. [DOI] [PubMed] [Google Scholar]
- Dell, K.R., and Williams, L.T. (1992). A novel form of fibroblast growth factor 2. Alternative splicing of the third immunoglobulin-like domain confers ligand binding specificity. J. Biol. Chem. 267, 21225-21229. [PubMed] [Google Scholar]
- Doble, B.W., Fandrich, R.R., Liu, L., Padua, R.R., and Kardami, E. (1990). Calcium protects pituitary basic fibroblast growth factors from limited proteolysis by co-purifying proteases. Biochem. Biophys. Res. Commun. 173, 1116-1122. [DOI] [PubMed] [Google Scholar]
- Drose, S., and Altendorf, K. (1997). Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200, 1-8. [DOI] [PubMed] [Google Scholar]
- Falnes, P.O., Wiȩdlocha, A., Rapak, A., and Olsnes, S. (1995). Farnesylation of CaaX-tagged diphtheria toxin A-fragment as a measure of transfer to the cytosol. Biochemistry 34, 11152-11159. [DOI] [PubMed] [Google Scholar]
- Florkiewicz, R.Z., Majack, R.A., Buechler, R.D., and Florkiewicz, E. (1995). Quantitative export of FGF-2 occurs through an alternative, energy-dependent, non-ER/Golgi pathway. J. Cell. Physiol. 162, 388-399. [DOI] [PubMed] [Google Scholar]
- Florkiewicz, R.Z., and Sommer, A. (1989). Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc. Natl. Acad. Sci. USA 86, 3978-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, R., Schmid, S., and Mellman, I. (1989). A possible role for Na+, K+-ATPase in regulating ATP-dependent endosome acidification. Proc. Natl. Acad. Sci. USA 86, 539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, A.M., Rowell, C., Ackermann, K., Kowalczyk, J.J., and Lewis, M.D. (1993). Peptidomimetic inhibitors of Ras farnesylation and function in whole cells. J. Biol. Chem. 268, 18415-18418. [PubMed] [Google Scholar]
- Gospodarowicz, D. (1987). Isolation and characterization of acidic and basic fibroblast growth factor. Methods Enzymol. 147, 106-119. [DOI] [PubMed] [Google Scholar]
- Hurley, M.M., Abreu, C., Gronowicz, G., Kawaguchi, H., and Lorenzo, J. (1994). Expression and regulation of basic fibroblast growth factor mRNA levels in mouse osteoblastic MC3T3-E1 cells. J. Biol. Chem. 269, 9392-9396. [PubMed] [Google Scholar]
- Imamura, T. et al. (1990). Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science 249, 1567-1570. [DOI] [PubMed] [Google Scholar]
- Jaakkola, P., Määttä, A., and Jalkanen, M. (1998). The activation and composition of FiRE (an FGF-inducible response element) differ in a cell type- and growth factor-specific manner. Oncogene 17, 1279-1286. [DOI] [PubMed] [Google Scholar]
- Klingenberg, O., Wiȩdlocha, A., Rapak, A., Munoz, R., Falnes, P., and Olsnes, S. (1998). Inability of the acidic fibroblast growth factor mutant K132E to stimulate DNA synthesis after translocation into cells. J. Biol. Chem. 273, 11164-11172. [DOI] [PubMed] [Google Scholar]
- Klingenberg, O., Wiȩdlocha, A., Citores, L., and Olsnes, S. (2000). Requirement of phosphatidylinositol 3-kinase activity for translocation of exogenous aFGF to the cytosol and nucleus. J. Biol. Chem. 275, 11972-11980. [DOI] [PubMed] [Google Scholar]
- Knee, R.S., Pitcher, S.E., and Murphy, P.R. (1994). Basic fibroblast growth factor sense (FGF) and antisense (gfg) RNA transcripts are expressed in unfertilized human oocytes and in differentiated adult tissues. Biochem. Biophys. Res. Commun. 205, 577-583. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Malecki, J., Wiȩdlocha, A., Wesche, J., and Olsnes, S. (2002). Vesicle transmembrane potential is required for translocation to the cytosol of externally added FGF-1. EMBO J. 21, 4480-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, I.J. (1994). The ins and outs of fibroblast growth factors. Cell 78, 547-552. [DOI] [PubMed] [Google Scholar]
- Miki, T., Bottaro, O.P., Fleming, T.P., Smith, C.L., Burgess, W.H., Chan, A.M., and Aaronson, S.A. (1992). Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc. Natl. Acad. Sci. USA 89, 248-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, Y., Kihara, K., Mizuno, K., Masamune, Y., Yoshitake, Y., and Nishikawa, K. (1992). Direct effect of basic fibroblast growth factor on gene transcription in cell-free system. Proc. Natl. Acad. Sci. USA 89, 5216-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes, S., Klingenberg, O., and Wiȩdlocha, A. (2003). Transport of exogenous growth factors and cytokines to the cytosol and to the nucleus. Physiol. Rev. 83, 163-182. [DOI] [PubMed] [Google Scholar]
- Ornitz, D.M. and Itoh, N. (2001) Fibroblast growth factors. Genome. Biol. 2, 3005.1-3005.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz, D.M., and Leder, P. (1992). Ligand specificity and heparin dependence of fibroblast growth factor receptors 1 and 3. J. Biol. Chem. 267, 16305-16311. [PubMed] [Google Scholar]
- Peng, H. et al. (2001). Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Mol. Biol. Cell 12, 449-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperkok, R., Lorenz, P., Ansorge, W., and Pyerin, W. (1994). Casein kinase II is required for transition of G0/G1, early G1, and G1/S phases of the cell cycle. J. Biol. Chem. 269, 6986-6991. [PubMed] [Google Scholar]
- Powers, C.J., McLeskey, S.W., and Wellstein, A. (2000). Fibroblast growth factors, their receptors and signaling. Endocrine-Related Cancer 7, 165-197. [DOI] [PubMed] [Google Scholar]
- Quarto, N., Finger, F.P., and Rifkin, D.B. (1991). The NH2-terminal extension of high molecular weight bFGF is a nuclear targeting signal. J. Cell. Physiol. 147, 311-318. [DOI] [PubMed] [Google Scholar]
- Reiss, Y., Goldstein, J.L., Seabra, M.C., Casey, P.J., and Brown, M.S. (1990). Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell 62, 81-88. [DOI] [PubMed] [Google Scholar]
- Renko, M., Quarto, N., Morimoto, T., and Rifkin, D.B. (1990). Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J. Cell. Physiol. 144, 108-114. [DOI] [PubMed] [Google Scholar]
- Shen, B., Arese, M., Gualandris, A., and Rifkin, D.B. (1998). Intracellular association of FGF-2 with ribosomal protein L6/TAXREB107. Biochem. Biophys. Res. Commun. 252, 524-528. [DOI] [PubMed] [Google Scholar]
- Sinensky, M., Fantle, K., Trujillo, M., McLain, T., Kupfer, A., and Dalton, M. (1994). The processing pathway of prelamin A. J. Cell Sci. 107, 61-67. [DOI] [PubMed] [Google Scholar]
- Skjerpen, C.S., Nilsen, T., Wesche, J., and Olsnes, S. (2002). Binding of FGF-1 variants to protein kinase CK2 correlates with mitogenicity. EMBO J. 21, 4058-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperinde, G.V., and Nugent, M.A. (2000). Mechanisms of fibroblast growth factor 2 intracellular processing: a kinetic analysis of the role of heparan sulfate proteoglycans. Biochemistry 39, 3788-3796. [DOI] [PubMed] [Google Scholar]
- Spivak-Kroizman, T. et al. (1994). Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell 79, 1015-1024. [DOI] [PubMed] [Google Scholar]
- Szebenyi, G., and Fallon, J.F. (1999). Fibroblast growth factors as multifunctional signaling factors. Int. Rev. Cytol. 185, 45-106. [DOI] [PubMed] [Google Scholar]
- Van den Berghe, K., Laurell, H., Huez, I., Zanibellato, C., Prats, H., and Bugler, B. (2000). FIF [Fibrobalst growth factor-2 (FGF-2)-interacting-factor], a putative antiapoptotic factor, interacts specifically with FGF-2. Mol. Endocrinol. 14, 1709-1724. [DOI] [PubMed] [Google Scholar]
- Wiȩdlocha, A., Falnes, P.O., Madshus, I.H., Sandvig, K., and Olsnes, S. (1994). Dual mode of signal transduction by externally added acidic fibroblast growth factor. Cell 76, 1039-1051. [DOI] [PubMed] [Google Scholar]
- Wiȩdlocha, A., Falnes, P.O., Rapak, A., Klingenberg, O., Munoz, R., and Olsnes, S. (1995). Translocation to cytosol of exogenous, CAAX-tagged acidic fibroblast growth factor. J. Biol. Chem. 270, 30680-30685. [DOI] [PubMed] [Google Scholar]
- Wiȩdlocha, A., Falnes, P.O., Rapak, A., Munoz, R., Klingenberg, O., and Olsnes, S. (1996). Stimulation of proliferation of a human osteosarcoma cell line by exogenous acidic fibroblast growth factor requires both activation of receptor tyrosine kinase and growth factor internalization. Mol. Cell. Biol. 16, 270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon, A., Zimmer, Y., Shen, G. H., Avivi, A., Yarden, Y., and Givol. D. (1992). A confined variable region confers ligand specificity on fibroblast growth factor receptors: implications for the origin of the immunoglobulin fold. EMBO J. 11, 1885-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, X., Hu, X., Friesel, R., and Maciag, T. (1993). Long term growth factor exposure and differential tyrosine phosphorylation are required for DNA synthesis in Balb/c 3T3 cells. J. Biol. Chem. 268, 9611-9620. [PubMed] [Google Scholar]