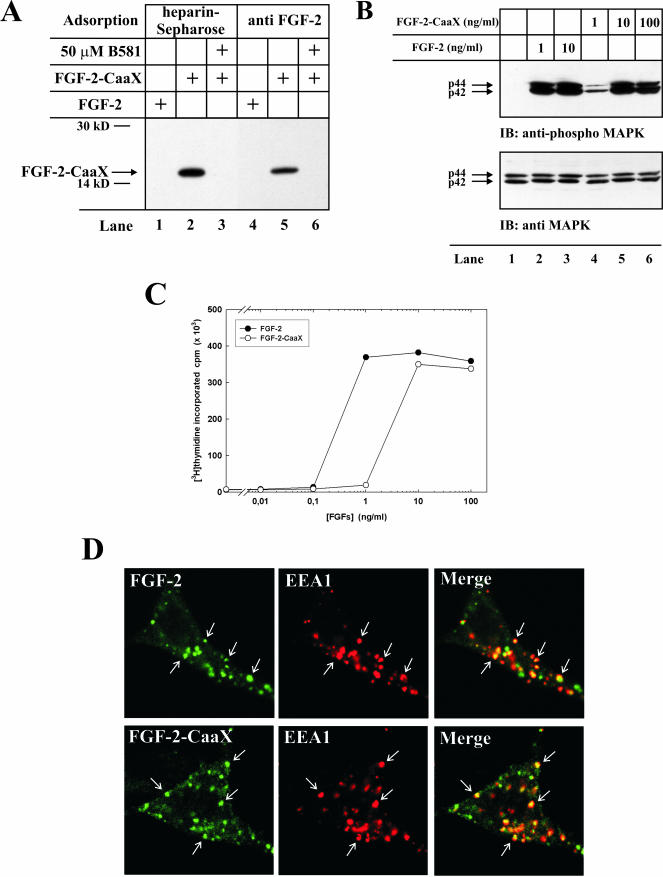
Figure 1.
In vitro farnesylation and biological activity of FGF-2-CaaX. (A) Recombinant FGF-2 or FGF-2-CaaX were incubated in a reticulocyte lysate system in the presence of [3H]farnesyl pyrophosphate in the absence or presence of B581. Samples were treated with heparin-Sepharose (lanes 1-3) or subjected to immunoprecipitation using anti-FGF-2 antibodies adsorbed to protein A-Sepharose (lanes 4-6) and analyzed by SDS-PAGE and fluorography. The arrow indicates the migration of farnesylated FGF-2-CaaX. (B) Serum-starved NIH/3T3 cells were treated with heparin and the indicated amount of FGF-2 or FGF-2-CaaX for 10 min. The cells were lysed in SDS sample buffer, sonicated, and analyzed by SDS-PAGE and Western blotting using antibodies against total p44/42 MAP kinase (bottom panel). The membrane was stripped and reprobed using antibodies against the phosphorylated form of p44/42 MAP kinase (top panel). (C) Serum-starved NIH/3T3 cells were treated with heparin and increasing amounts of FGF-2 or FGF-2-CaaX for 24 h. During the last 6 h of incubation, 1 μCi/ml [3H]thymidine was present in the medium. The cells were extracted with 5% TCA and the radioactivity incorporated into TCA-insoluble material was measured. (D) FGF-2 or FGF-2-CaaX conjugated to Alexa 488 maleimide was added to NIH/3T3 cells transfected with pcDNA3 vector encoding FGFR1 and incubated for 15 min at 37°C. The cells were then fixed, permeabilized, and treated with a mouse antibody against EEA1. The cells were further treated with Cy3-conjugated anti-mouse antibody. The arrows point to examples of structures positive for EEA1 and FGF-2 or FGF-2-CaaX.