Abstract
Objective
The complex movement of the temporomandibular joint (TMJ) disc during mastication is controlled in large part by the disc’s attachments to the surrounding tissues. This study seeks to address the lack of available quantitative data characterizing the extracellular matrix composition of the discal attachments and how these properties compare to the disc.
Design
Porcine TMJ disc-attachment complexes were carefully dissected into six discal attachments and five TMJ disc regions. All samples were assayed biochemically for total collagen, glycosaminoglycan (GAG), DNA, and hydration. Additionally, histology was performed on the whole joint to investigate the anatomy of the disc-attachment complex, and to verify the regional distribution of matrix components.
Results
Quantitative biochemical assays showed that overall water content was fairly constant in all disc and attachment regions. Disc regions generally showed higher sulfated GAG and collagen content than the attachments. In contrast, the attachments contained greater DNA content than the disc. Histological staining supported the quantitative results and also indicated more elastic fibers to be present in the attachments than the disc.
Conclusions
Although macroscopically the TMJ disc and its attachments form a seamless complex within the joint, a closer look at regional biochemical constituents reveals that these two components are distinct. While the disc and attachments both contain the same major constituents, the relative amounts of these components vary based on the functional requirements of the tissue. These results can further understanding of both TMJ biology and pathology.
Keywords: Temporomandibular Joint, Disc, Attachments, Tissue Engineering, Collagen, Glycosaminoglycan, DNA, Elastin
Introduction
The temporomandibular joint (TMJ) is a hinge joint that allows for normal opening and closing of the mandible, and is essential for everyday functions of the mouth such as mastication and speaking. It is comprised of the superior (glenoid fossa) and inferior (mandibular condyle) articulating surfaces, and a fibrocartilaginous disc suspended between them which helps align and reduce friction in the joint.1 However, this joint is prone to a variety of pathologies that inhibit normal jaw function that manifest through pain, tissue degeneration, and displacement of the TMJ disc. Collectively, temporomandibular joint disorders (TMDs) cause loss of jaw function and affect millions of people in the United States.2 Unfortunately, the causes of TMD are ill-understood and current clinical therapies are limited to managing the painful symptoms of the disease.3 In extreme cases tissue resection is performed to alleviate discomfort, but this course of action is not optimal as it is often followed by further deterioration and joint degeneration.4
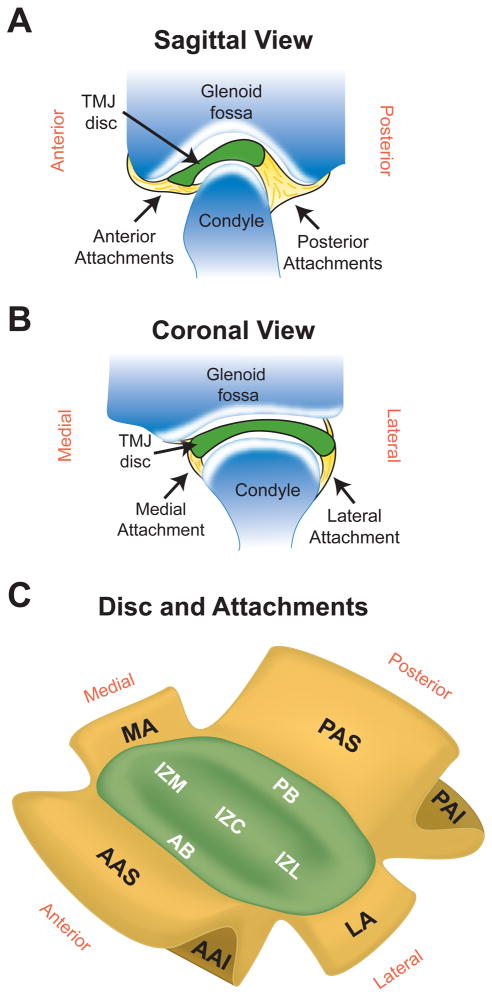
Regenerative medicine efforts to recapitulate the complex biochemistry and biomechanics of the TMJ disc are currently underway, and may provide a possible clinical alternative to tissue resection. However, if a suitable disc replacement is engineered, how it will be attached within the joint will need to be carefully considered. In the native joint, the attachments of the TMJ disc with the surrounding tissues are extremely important for the coordinated movements of the TMJ.5 A detailed anatomy of the attachments can be found in the literature.5–7 Briefly, the anterior portion of the disc attaches inferiorly to the anterior condyle and superiorly to the eminence by bending with the joint capsule (Fig 1A). Posteriorly, the disc attaches superiorly to the temporal bone and inferiorly to the posterior condyle (the posterior attachments are frequently called the bilaminar zone). Laterally and medially, the disc attachments blend into the joint capsule near its attachment to the condylar head (Fig 1B).
Figure 1.
Anatomy and regions of the TMJ disc and its attachments. (A) Sagittal view of the TMJ showing the anterior and posterior discal attachments which both bifurcate into superior and anterior attachments. (B) Coronal view of the TMJ detailing the medial and lateral attachments which both blend into the joint capsule near its attachment to the condyle. (C) Depiction of the 5 disc regions and 6 discal attachments analyzed in this study, which span the joint in the anteroposterior and mediolateral directions.
Although the discal attachments are vital for proper movement and health of the TMJ disc, currently little is known about the quantitative properties of these tissues. Histological studies have revealed that there is a general lack of chondroitin sulfate glycosaminoglycans (GAGs) and collagen type II in the attachments,8–10 indicating that these tissues are not fibrocartilaginous like the disc. The lack of cartilaginous phenotype in the attachments is supported by the cell morphology in these tissues, which is entirely fibroblastic.11 Polarized light microscopy and scanning electron microscopy (SEM) have shown that collagen fibrils extend from the disc into the attachments and are particularly dense in the posterior attachment.12, 13 Elastin staining can also be found throughout the attachments and is more abundant than in the disc.14 While these studies provide an excellent starting point for understanding the attachments, complete quantitative analysis is needed to fully understand the role of each discal attachment.
With regards to the TMJ disc, its biochemical content and distribution has been described quite extensively. The disc is composed primary of collagen, comprising 83–96% of the dry weight.15, 16 Collagen concentration is highest in the bands of the disc relative to lateral region.16 A wide variety of values have been reported for the total amount of GAGs in the TMJ disc, but the general consensus is around 1% of the dry weight.2 Studies indicate that the greatest GAG content is located in the center of the disc relative to the bands.16, 17 Histological studies of the pig disc indicate that approximately 70% of the cells in the disc are fibroblastic in morphology, with the remainder displaying a round chondrocyte morphology.18 Cellular density is highest in the anterior and posterior bands.8, 16
Although the disc attachments are an integral part of the TMJ, little is still known about the exact biochemistry of these tissues and how they compare to the TMJ disc itself. Therefore, this study seeks to characterize the anteroposterior and mediolateral disc-attachment complex biochemically and histologically. It is hypothesized that the attachments of the disc will show biochemical similarity to the disc itself, but that regional variations in biochemical content will be observed. The major findings of this study will help to identify the relative roles of disc and attachment in TMJ physiology and disease.
Materials and Methods
Specimen Procurement
Porcine heads from female animals 6–9 months of age were obtained from a local abattoir (Yosemite Meat Co., Modesto, CA). A porcine model was used because the joint kinematics and discal properties of the porcine TMJ are similar to the human joint.19–22 The entire TMJ and its surrounding bony structures (e.g., condylar process, temporal bone and zygomatic arch) were removed en bloc using an osteotome and mallet. All to the soft tissues of the joint were dissected from the bone using a scalpel and periosteal elevator. During the procedure a periodic irrigation with PBS solution was used to avoid drying the specimen. Following isolation of the TMJ soft tissues, muscular and adipose tissues were carefully dissected away leaving an intact disc-attachment complex. Gross inspection of all samples did not reveal any degeneration.
Biochemical Analysis
After isolation, TMJ discs were dissected into five regions and discal attachments were dissected into six regions as shown in Fig. 1B. The five regions of the disc tested were: posterior band (PB), anterior band (AB), intermediate zone medial (IZM), intermediate zone central (IZC), and intermediate zone lateral (IZL). The six discal attachments examined were: posterior attachment superior (PAS), posterior attachment inferior (PAI), anterior attachment superior (AAS), anterior attachment inferior (AAI), medial attachment (MA), and lateral attachment (LA). Samples were taken from the center of each attachment at the four poles of the joint. For the branching attachments (AA and PA), the portion of the attachment that was closest to the interior of the joint was collected for analysis. Following dissection, samples were blotted dry and wet weights were measured. Samples were frozen for 24 hrs and lyophilized for 48 hrs before dry weights were taken. Digestion occurred in a 125 mg/mL papain (Sigma, St. Louis, MO) solution overnight at 60°C. At the end of digestion, no residual tissue remained. DNA content was measured with the Quant-iT Picrogreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA). Following hydrolysis with 4 N NaOH for 20 min at 110°C, collagen content of the samples was quantified with a modified chloramine-T hydroxyproline assay.16 Finally, sulfated GAG content was quantified using Blyscan Glycosaminoglycan Assay Kit (Accurate Chemical and Scientific Corp., Westbury, NY). n = 6 samples per group was used for all biochemical analysis.
Histology
Two left joints en bloc were trimmed and fixed in 10% neutral buffered formalin for one wk. Joints were decalcified using 10% formic acid and cut into regional sections using a scalpel blade. Each joint was cut into three pieces: 1) sagittal through the center of the entire joint, 2) coronal through the medial side of the joint, 3) coronal through the lateral side of the joint. Samples were embedded in paraffin wax and sectioned at 5 μm. Cellular distribution and general matrix compositions were investigated with hematoxylin and eosin (H & E) staining. Alcian blue staining at pH 2.5 was used to examine the distribution of sulfated GAGs. Collagen and elastin were examined with Verhoeff’s Van Gieson staining.
Statistical Analysis
All quantitative results were compared using a one-way analysis of variance (ANOVA). A Tukey’s HSD post hoc test was used where appropriate. A significance level of p < 0.05 was used for all statistical analysis.
Results
Biochemical Analysis
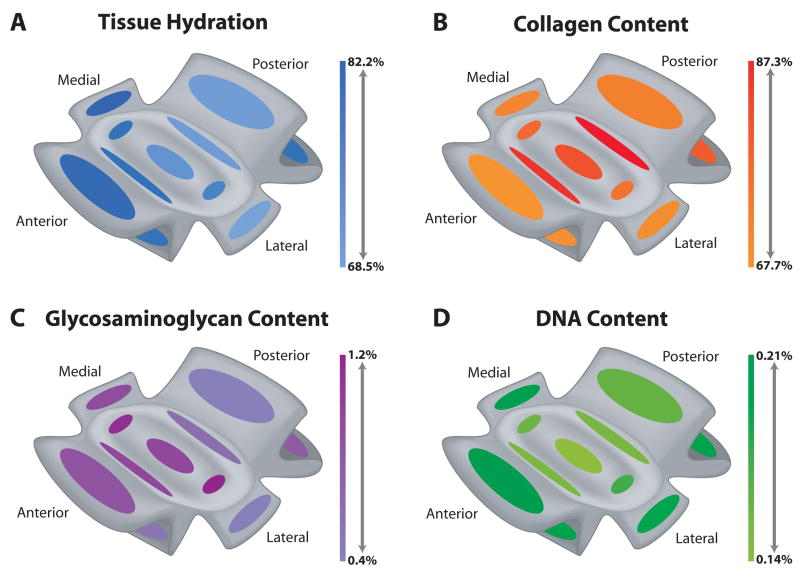
Biochemical results for the TMJ disc and its attachments are shown pictorially in Fig. 2 and the raw data can be found in Table 1. The biochemical content for the disc was similar to that measured in prior studies.16, 17, 19
Figure 2.
Heat maps of biochemical content throughout the TMJ disc and its attachments. Mean content normalized to dry weight for each region is presented as color intensity in the scale to the right of each picture. The top and bottom of the scale represent the highest and lowest mean value for each parameter. (A) Water content was highest in anterior and medial attachments with few large variations. (B) Collagen content was highest in the disc compared to the attachments, particularly in the bands of the disc. (C) Overall, the disc contained more sulfated GAG than the attachments, although the medial attachment and the superior portion of the anterior attachment did contain a significant amount of GAG. (D) DNA per dry weight was generally higher in the attachments than the disc, except in the superior portion of the posterior band.
Table 1.
Quantitative results for the biochemical content of the TMJ disc and its attachments. Data is presented at mean ± SD. ANOVA results presented are from a one-way with a Tukey’s HSD post hoc test. Groups not connected by the same letter are statically different from each other. Water content did not vary greatly, but was highest in MA and lowest in LA. PB contained the highest collagen per dry weight, while AAS contained the least. Sulfated GAG content was statically higher in IZM and IZL, while PAS had statically less. DNA per dry weight was greatest in AAS and MA, whereas it was the least in IZC.
| Water Content (%) | Collagen/Dry Weight (%) | sGAG/Dry Weight (%) | DNA/Dry Weight (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | Region | Mean ± SD | ANOVA | Mean ± SD | ANOVA | Mean ± SD | ANOVA | Mean ± SD | ANOVA |
| Attachment | PAS | 68.81 ± 2.86 | BC | 71.75 ± 7.08 | AB | 0.4 ± 0.11 | D | 0.16 ± 0.03 | BCD |
| PAI | 73.76 ± 1.78 | BC | 78.9 ± 10.08 | AB | 0.76 ± 0.18 | ABCD | 0.19 ± 0.01 | AB | |
| AAS | 80.35 ± 2.39 | AB | 67.72 ± 13.96 | B | 0.82 ± 0.2 | ABC | 0.21 ± 0 | A | |
| AAI | 73.2 ± 4.06 | BC | 68.45 ± 8.1 | AB | 0.5 ± 0.14 | CD | 0.2 ± 0.01 | AB | |
| MA | 82.15 ± 1.19 | A | 71.16 ± 9.26 | AB | 0.88 ± 0.16 | ABC | 0.2 ± 0.01 | A | |
| LA | 68.49 ± 4.44 | C | 69.27 ± 12.38 | AB | 0.4 ± 0.28 | CD | 0.19 ± 0.03 | ABC | |
| Disc | PB | 69.09 ± 1.51 | BC | 87.3 ± 7 | A | 0.59 ± 0.07 | BCD | 0.15 ± 0.01 | CD |
| AB | 73.81 ± 4.95 | BC | 84.2 ± 12.13 | AB | 0.94 ± 0.23 | AB | 0.15 ± 0.03 | CD | |
| IZM | 73.22 ± 1.75 | BC | 76.36 ± 9.4 | AB | 1.11 ± 0.25 | A | 0.16 ± 0.01 | BCD | |
| IZC | 70.26 ± 1.87 | BC | 80.46 ± 7.15 | AB | 0.93 ± 0.13 | AB | 0.14 ± 0.02 | D | |
| IZL | 72.02 ± 3.94 | BC | 74.71 ± 4.79 | AB | 1.18 ± 0.36 | A | 0.18 ± 0.02 | ABCD | |
Water Content
Overall, the water content was quite similar among all tissues examined with most groups having a mean water content of ~73% (Fig. 2A, Table 1). Water content did not vary among the five regions of the TMJ disc measured. The discal attachments did show significant differences, with MA containing the most water at 82.2% and LA containing the least water at 68.5%. Water content of AAS was also high at 80.4%.
Distribution of Collagen
Similar to the water content, there were not many statistical differences among the disc and attachment regions. Overall, the disc had greater mean collagen content at 80.6%, compared to the attachments which had a mean of 71.2% (Fig. 2B, Table 1). Statistically, PB contained the most collagen per dry weight at 87.3%, but the other band of the disc, AB, was also high at 84.2%. AAS contained the least collagen content overall at 67.7%. The attachment with the most collagen per dry weight was PAI with a mean of 78.9%.
Distribution of Glycosaminoglycans
Sulfated GAG per dry weight showed a larger regional variation among the tissues than the other biochemical parameters. Overall, the TMJ disc had a higher mean GAG content at 0.95%, compared to the attachments which had a mean of 0.63% (Fig. 2C, Table 1). The one-way ANOVA indicated that IZM and IZL contained the most GAG per dry weight at 1.11% and 1.18%, respectively. The only region of the disc that did not contain a large amount of GAG was PB which had a mean of 0.59%. Statistically, the tissue with the least GAG was PAS at 0.40%, although AAI and LA were also low. The three attachments with the most GAG were PAI, AAS, and MA with contents of 0.76%, 0.82% and 0.88% respectively.
Distribution of DNA
DNA content normalized to dry weight also showed a distinct regional variation, although the variation was more prominent in the attachments. Overall, the attachments had a higher mean DNA content at 0.19%, compared to the disc which had a mean of 0.16% (Fig. 2D, Table 1). Statistically, AAS and MA contained the most DNA per dry weight at 0.21% and 0.20% respectively. The attachment with the least DNA was PAS at 0.16%. The one-way ANOVA indicated that the tissue with the least DNA was IZC at 0.14%. IZL was the only disc region with a high DNA content (0.18%).
Histological Analysis
Whole Joint Histology
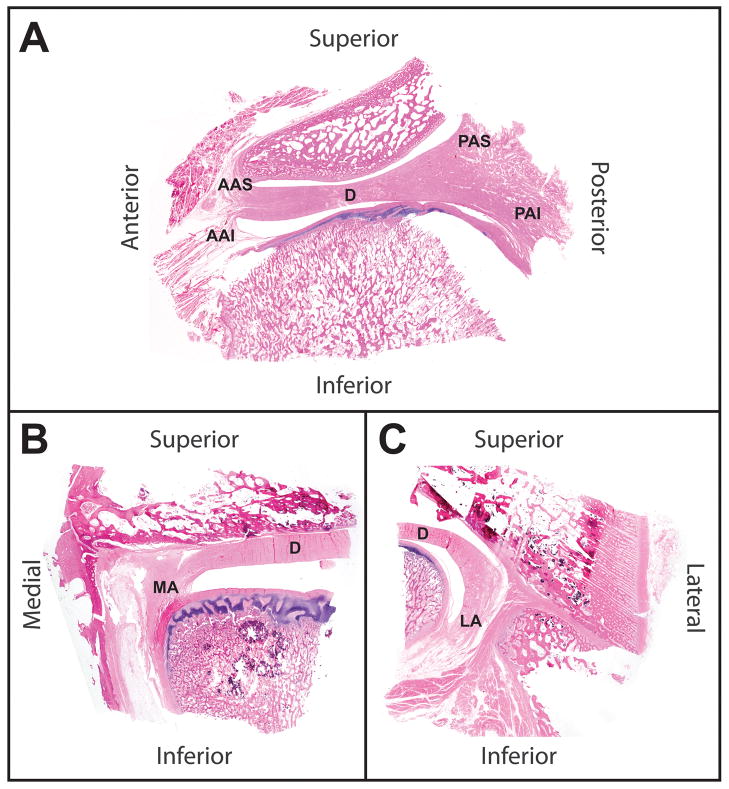
Whole joint histological staining was used to examine the anatomy of the discal attachments. In Fig. 3A, a sagittal view of the disc and its attachments shows that the anterior attachment is markedly smaller than the posterior attachment. The matrix of the posterior attachment appears to be a similar density as the TMJ disc itself, while the anterior attachment shows more diffuse fibers that run superiorly and inferiorly in the joint. The medial and lateral attachments (Fig. 3B and C) are similar to the anterior attachment in appearance, but these attachments join mainly with the inferior mandibular condyle. The medial attachment connects at the top of the condylar head, while the lateral attachment connects very low on the condylar neck.
Figure 3.
Whole joint histology of the TMJ. Sections were cut at 5 μm and stained with hematoxylin and eosin. (A) Anteriorly and posteriorly the disc (D) blends into the attachments which both bifurcate into superior and anterior boney connections. The disc has a denser matrix than the attachments. (B) In the medial portion of the TMJ, the disc blends into the medial attachment high in the joint and the attachment to the condyle is at the top of the condylar head. (C) On the medial side of the joint, the disc wraps around the side of the condylar head and the lateral discal attachment attaches to the condyle near its base.
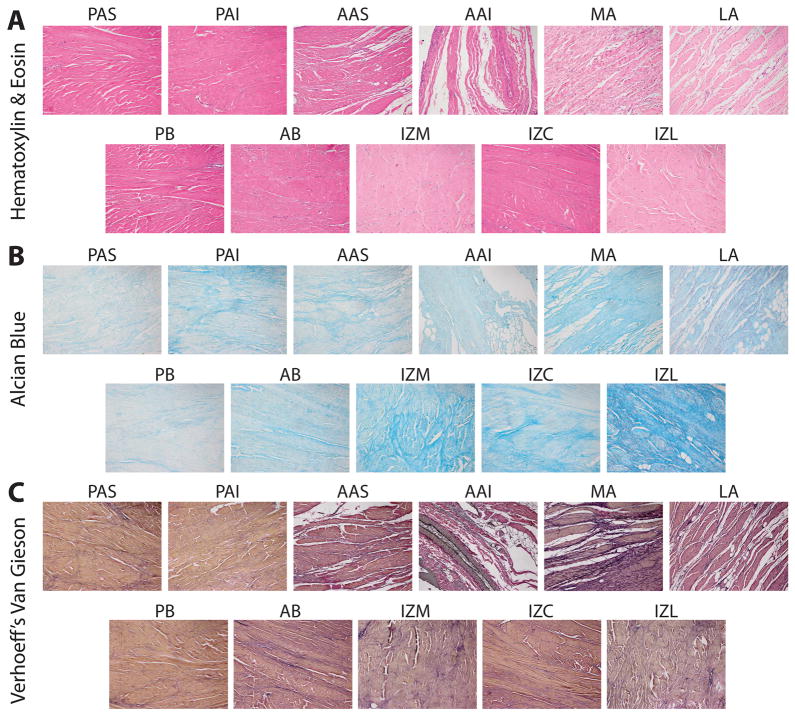
Regional Histology
Histological staining of the different regions of the disc and attachments showed some differences in matrix components (Fig. 4). As seen in the whole joint histology (Fig. 3), regional H & E staining of the disc-attachment complex indicate that the posterior attachment has a similar matrix density to the disc, and the anterior, medial, and lateral attachments have more diffuse matrices (Fig. 4A). Nuclear staining indicated higher cellular density in the attachment regions relative to the disc, supporting the quantitative DNA results. Alcian blue staining for regional GAG content was more apparent in the disc than in the attachments, though the attachments were not devoid of this component (Fig. 4B). Alcian blue staining was most intense in IZL and IZM, agreeing with the quantitative results. Verhoeff’s Van Gieson staining was positive for collagen and elastin throughout all regions of the disc attachment complex (Fig. 4C). Elastin staining was most prevalent in AAI and MA, and also revealed a large diameter blood vessel running through AAI.
Figure 4.
Regional histological staining of the TMJ disc and its attachments. In general, all histological staining verified quantitative results. (A) Hematoxylin and eosin illustrate that cellular density is higher in the attachments, while the disc contains a denser ECM than all of the attachments expect PAS and PAI. (B) Alcian blue staining clearly shows the higher sulfated GAG content of the TMJ disc in comparison to the attachments. IZM and IZL have the most GAG overall, while MA was the attachment with the most staining. (C) Verhoeff’s Van Gieson staining clearly shows collagen (red-brown) and elastic (black) fibers throughout the TMJ. PAS and PAI appeared to have similar content to the disc, but the other attachments displayed increased elastin staining.
Discussion
Although recent research has made significant advances to our understanding of TMJ disc structure and function, it is clear that there is dearth of information about the attachments that anchor the disc within the joint. Based on the quantitative biochemical evaluation carried out in this study, the discal attachments show many key similarities with the TMJ disc itself. They both contain the same basic components (collagen, GAG, cells, and elastin) and much of the matrix is continuous between the tissues, blending seamlessly together. Although vast differences were not found, the disc and attachments were found to be regionally distinct, and these distinctions are likely related to the functional requirements of each region.
Overall, DNA content was lower and GAG and collagen content were higher in the disc compared to the attachments. These distinctions confirm the previously described fibrocartilaginous nature of the disc and ligamentous properties of the discal attachments.8, 13 The most basic difference between these tissues is that fibrocartilage contains more GAG and the presence of collagen type II.2 Higher cellular density in the attachments may be attributed to two factors: 1) cellular density is typically higher in ligamentous tissues compared to cartilages,23 and 2) more vasculature was seen in the attachments. Although the sulfated GAG content of the disc and attachments are both quite low (≤1.2%), the fibrocartilaginous disc was found to contain more GAG overall. This is particularly true in the intermediate zone of the disc where higher GAG content is accompanied by more chondrocyte-like cells and collagen type II content.17, 18 The higher collagen content observed in the disc is not necessarily a trait of cartilaginous tissues, but is consistent with polarized light micrographs indicating that the disc possesses more aligned collagen fibers than the attachments.13 Additionally, the greater elastic fiber staining seen in the attachments likely reduces their relative collagen content. Although they are not vast, the biochemical distinctions between the disc and its attachments relate well to their tissue classifications, and also likely relate to functional properties.
To fully understand the functional role of the attachments and disc within the TMJ, it is important to relate the tissue’s biochemical composition with its biomechanical properties. While the mechanics of the TMJ disc have been well described, few studies have attempted mechanical characterization the discal attachments. Thus far, only the posterior attachment (retrodiscal tissue) has been thoroughly examined. Under compression, the posterior attachment was found to have an elastic modulus of 1.54 MPa,24 which was approximately 20 times less stiff than the disc.25 The large disparity in moduli between these tissues is likely due to the disc’s higher sulfated GAG content. Under tension, the posterior attachment has been shown to possess an elastic modulus of 4.30 MPa,26 approximately five times less than the corresponding modulus for the disc.27 The greater collagen content seen in the disc may provide a basis for the higher tensile modulus found in this tissue. Based on these results, it is prudent that future mechanical characterization of all attachments be performed so that structure-function relationships can be collectively drawn.
Regional variation in the discal attachments was present and was most obvious in sulfated GAG content. In the anteroposterior direction, the AAS and PAI had high mean GAG contents, while the AAI and PAS contained little GAG. Since sulfated GAG content is generally related to compressive requirements of the tissue, this variation can be logically described in terms of mastication. As the disc translates forward during jaw opening, the AAI is under tension at the front of the condyle, while the AAS gets compressed between the disc and fossa.28–30 In the posterior region of the joint, the opposite is true. Here, the PAS becomes stretched with disc translation, while the PAI is compressed beneath the PB.28–30 A similar argument may be made for the increased GAG content of the MA versus the LA. The MA attaches high on the condylar head (Fig. 3B) and is clearly within the articulating surface. As a result, it likely experiences more compressive loading than the LA, which attaches low on the side of the condylar head (Fig. 3C). Water content is generally correlated with sulfated GAG content because the negative charges draw in water molecules.31 This trend was accurately seen within the attachments. Collagen content did not vary greatly among the attachments, although the PAI had the highest mean content. This is consistent with a prior report indicating that the PAI contained the largest number of collagen fiber bundles.13
Understanding the salient characteristics of the attachments is essential not only in terms of elucidating structure-function relationships in the TMJ in vivo, but also toward establishing approaches for implanting TMJ grafts or tissue engineered constructs. The knowledge gained from the whole joint histology performed in the present study provides important guides for identifying the appropriate anatomical attachment locations for engineered TMJ discs. Furthermore, future tissue engineering efforts are likely to benefit from this study’s finding that the biochemical components of both disc and attachments are similar. It appears that the main distinction between these tissues is that the disc is a fibrocartilage, while the attachments are generally fibrous tissues. Although these tissues are distinct, they are similar enough that it may be possible for tissue engineers to generate them using the same cell population. Literature on tendon tissue engineering has demonstrated that starting with a fibroblast cell population it is possible to produce both fibrous and fibrocartilaginous tissues through variation of the cell’s local mechanical environment.32–34 It may also be possible to engineer the disc-attachment complex with cartilaginous cells, as chondrocytes exposed to cyclic tension take on a fibroblastic phenotype.35–37 Thus, construction of a tension-compression bioreactor for recapitulating the regional variation of the disc-attachment complex would likely aid in the development of engineered TMJ complex replacements.
Knowledge of the similarities and differences between the TMJ disc and its attachments is crucial for understanding TMJ biology and TMD pathologies, as well as developing tissue engineered replacements. While additional mechanical characterization is needed to fully understand the structure-function relationships within the attachments and disc, the quantitative biochemical parameters presented here are a crucial first step toward this goal.
Acknowledgments
The authors gratefully acknowledge funding support from NIDCR R01DE015038.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanaka E, van Eijden T. Biomechanical behavior of the temporomandibular joint disc. Crit Rev Oral Biol Med. 2003;14(2):138–150. doi: 10.1177/154411130301400207. [DOI] [PubMed] [Google Scholar]
- 2.Allen KD, Athanasiou KA. Tissue engineering of the TMJ disc: a review. Tissue Eng. 2006;12(5):1183–1196. doi: 10.1089/ten.2006.12.1183. [DOI] [PubMed] [Google Scholar]
- 3.Wong ME, Allen KD, Athanasiou KA. Biomedical engineering handbook. Boca Raton: CRC Press; 2006. Tissue engineering of the temporomandibular joint; pp. 52–51–52–22. [Google Scholar]
- 4.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87(4):296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 5.Rees LA. The Structure and function of the mandibular joint. Br Dent J. 1954;96:125–133. [Google Scholar]
- 6.Piette E. Anatomy of the human temporomandibular joint. An updated comprehensive review. Acta Stomatol Belg. 1993;90(2):103–127. [PubMed] [Google Scholar]
- 7.Christo JE, Bennett S, Wilkinson TM, Townsend GC. Discal attachments of the human temporomandibular joint. Aust Dent J. 2005;50(3):152–160. doi: 10.1111/j.1834-7819.2005.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 8.Mills DK, Fiandaca DJ, Scapino RP. Morphologic, microscopic, and immunohistochemical investigations into the function of the primate TMJ disc. J Orofac Pain. 1994;8(2):136–154. [PubMed] [Google Scholar]
- 9.Kopp S. Topographical distribution of sulphated glycosaminoglycans in human temporomandibular joint disks. A histochemical study of an autopsy material. J Oral Pathol. 1976;5(5):265–276. doi: 10.1111/j.1600-0714.1976.tb01775.x. [DOI] [PubMed] [Google Scholar]
- 10.Kondoh T, Hamada Y, Iino M, Takahashi T, Kikuchi T, Fujikawa K, et al. Regional differences of type II collagen synthesis in the human temporomandibular joint disc: immunolocalization study of carboxy-terminal type II procollagen peptide (chondrocalcin) Arch Oral Biol. 2003;48(9):621–625. doi: 10.1016/s0003-9969(03)00067-0. [DOI] [PubMed] [Google Scholar]
- 11.Paegle DI, Holmlund AB, Reinholt FP. Characterization of tissue components in the temporomandibular joint disc and posterior disc attachment region: internal derangement and control autopsy specimens compared by morphometry. J Oral Maxillofac Surg. 2002;60(9):1032–1037. doi: 10.1053/joms.2002.34416. [DOI] [PubMed] [Google Scholar]
- 12.Benigno MI, Azeredo RA, Lemos JL, Konig Junior B, Liberti EA. The structure of the bilaminar zone in the human temporomandibular joint: a light and scanning electron microscopy study in young and elderly subjects. J Oral Rehabil. 2001;28(2):113–119. doi: 10.1046/j.1365-2842.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 13.Scapino RP, Obrez A, Greising D. Organization and function of the collagen fiber system in the human temporomandibular joint disk and its attachments. Cells Tissues Organs. 2006;182(3–4):201–225. doi: 10.1159/000093969. [DOI] [PubMed] [Google Scholar]
- 14.Clement C, Bravetti P, Plenat F, Foliguet B, Haddioui AE, Gaudy JF, et al. Quantitative analysis of the elastic fibres in the human temporomandibular articular disc and its attachments. Int J Oral Maxillofac Surg. 2006;35(12):1120–1126. doi: 10.1016/j.ijom.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Nakano T, Scott PG. A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch Oral Biol. 1989;34(9):749–757. doi: 10.1016/0003-9969(89)90082-4. [DOI] [PubMed] [Google Scholar]
- 16.Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical analysis of the porcine temporomandibular joint disc. Br J Oral Maxillofac Surg. 2006;44(2):124–128. doi: 10.1016/j.bjoms.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005;24(1):45–57. doi: 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, et al. Cell type and distribution in the porcine temporomandibular joint disc. J Oral Maxillofac Surg. 2006;64(2):243–248. doi: 10.1016/j.joms.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. An interspecies comparison of the temporomandibular joint disc. J Dent Res. 2011;90(2):193–198. doi: 10.1177/0022034510381501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermejo A, Gonzalez O, Gonzalez JM. The pig as an animal model for experimentation on the temporomandibular articular complex. Oral Surg Oral Med Oral Pathol. 1993;75(1):18–23. doi: 10.1016/0030-4220(93)90399-o. [DOI] [PubMed] [Google Scholar]
- 21.Herring SW. TMJ anatomy and animal models. J Musculoskelet Neuronal Interact. 2003;3(4):391–394. discussion 406–397. [PMC free article] [PubMed] [Google Scholar]
- 22.Herring SW, Rafferty KL, Liu ZJ, Marshall CD. Jaw muscles and the skull in mammals: the biomechanics of mastication. Comp Biochem Physiol A Mol Integr Physiol. 2001;131(1):207–219. doi: 10.1016/s1095-6433(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 23.Shortkroff S, Spector M. Isolation and in vitro proliferation of chondrocytes, tenocytes, and ligament cells. Methods Mol Med. 1999;18:195–203. doi: 10.1385/0-89603-516-6:195. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka E, Del Pozo R, Sugiyama M, Tanne K. Biomechanical response of retrodiscal tissue in the temporomandibular joint under compression. J Oral Maxillofac Surg. 2002;60(5):546–551. doi: 10.1053/joms.2002.31853. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka E, Tanaka M, Miyawaki Y, Tanne K. Viscoelastic properties of canine temporomandibular joint disc in compressive load-relaxation. Arch Oral Biol. 1999;44(12):1021–1026. doi: 10.1016/s0003-9969(99)00097-7. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka E, Hanaoka K, Tanaka M, Van Eijden T, Iwabe T, Ishino Y, et al. Viscoelastic properties of bovine retrodiscal tissue under tensile stress-relaxation. Eur J Oral Sci. 2003;111(6):518–522. doi: 10.1111/j.0909-8836.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka E, Tanaka M, Hattori Y, Aoyama J, Watanabe M, Sasaki A, et al. Biomechanical behaviour of bovine temporomandibular articular discs with age. Arch Oral Biol. 2001;46(11):997–1003. doi: 10.1016/s0003-9969(01)00072-3. [DOI] [PubMed] [Google Scholar]
- 28.Scapino RP. The posterior attachment: its structure, function, and appearance in TMJ imaging studies. Part 1. J Craniomandib Disord. 1991;5(2):83–95. [PubMed] [Google Scholar]
- 29.Scapino RP. The posterior attachment: its structure, function, and appearance in TMJ imaging studies. Part 2. J Craniomandib Disord. 1991;5(3):155–166. [PubMed] [Google Scholar]
- 30.Westesson PL, Kurita K, Eriksson L, Katzberg RW. Cryosectional observations of functional anatomy of the temporomandibular joint. Oral Surg Oral Med Oral Pathol. 1989;68(3):247–251. doi: 10.1016/0030-4220(89)90203-x. [DOI] [PubMed] [Google Scholar]
- 31.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–256. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 32.Li KW, Lindsey DP, Wagner DR, Giori NJ, Schurman DJ, Goodman SB, et al. Gene regulation ex vivo within a wrap-around tendon. Tissue Eng. 2006;12(9):2611–2618. doi: 10.1089/ten.2006.12.2611. [DOI] [PubMed] [Google Scholar]
- 33.Spalazzi JP, Vyner MC, Jacobs MT, Moffat KL, Lu HH. Mechanoactive scaffold induces tendon remodeling and expression of fibrocartilage markers. Clin Orthop Relat Res. 2008;466(8):1938–1948. doi: 10.1007/s11999-008-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J Anat. 1998;193 (Pt 4):481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderploeg EJ, Imler SM, Brodkin KR, Garcia AJ, Levenston ME. Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech. 2004;37(12):1941–1952. doi: 10.1016/j.jbiomech.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 36.Connelly JT, Vanderploeg EJ, Levenston ME. The influence of cyclic tension amplitude on chondrocyte matrix synthesis: experimental and finite element analyses. Biorheology. 2004;41(3–4):377–387. [PubMed] [Google Scholar]
- 37.Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem. 1999;125(5):966–975. doi: 10.1093/oxfordjournals.jbchem.a022376. [DOI] [PubMed] [Google Scholar]