Abstract
Objectives
Tissue resident stem cells are believed to exist in most tissues, and their identification is commonly done by a combination of immunostaining for putative stem cell markers and the label-retaining cell (LRC) strategy. In the present study we employed these approaches to identify potential stem cells in the urinary bladder.
Methods
Newborn rats were intraperitoneally injected with 5-ethynyl-2-deoxyuridine (EdU), and their bladders harvested at four different time points afterward. The bladders were processed for EdU staining and immunofluorescence staining for stem cell markers Lgr5, CD34, SSEA-1, and c-kit. EdU-positive cells were counted and co-localization with stem cell markers determined.
Results
At day one post-EdU injection, 1804.0 ± 227.7 bladder cells were labeled in each cross section. As time increased, fewer bladders remained labeled, dropping to 236.5±53.0 cells per field. In the 1-day bladders, 27.5±4.9% of the epithelial cells were labeled as compared to 12.1±2.8% in the detrusor. The labeling rates in these two tissue compartments gradually equalized, reaching at approximately 5.5% in the 8-week samples. Distribution of LRC was random, without preferential labeling of basal cells. Lgr5 and SSEA-1 were detectable in the urothelium; CD34 and c-kit in the lamina propria and detrusor. Approximately 30 to 40% of c-kit-positive cells were EdU-positive.
Conclusions
Labeling of bladder cells by EdU occurred randomly, and label retaining was not associated with expression of Lgr5, CD34, or SSEA-1. The strong association between label retaining and c-kit expression appears to relate to interstitial cells of Cajal (ICCs), not stem cells.
Keywords: urinary bladder, EdU labeling, label-retaining cells, stem cell markers, c-kit, interstitial cells of Cajal
INTRODUCTION
It is generally believed that tissue-specific stem cells exist in most mammalian tissues 1,2, and their function is to maintain tissue homeostasis by supplying new tissue-specific cells during normal tissue cycling and when existing tissue cells are lost due to injuries 2. There is also evidence that these cells may be the origin of tumors and are thus potential therapeutic targets 3,4. To identify such cells various approaches have been employed, including the use of semi-specific stem cell markers. One of such markers is leucine-rich G protein-coupled receptor 5 (Lgr5) that is expressed in putative stem cells of the gastrointestinal epithelium 5. Lgr5 is also expressed in putative hair follicle stem cells 6 and potentially in other epithelial stem cells as well. CD34 has been used as a marker for mesenchymal stem cells (MSCs), but its expression in non-stem cells such as endothelial cells can complicate data interpretation 7. Stage-specific embryonic antigen 1 (SSEA-1) is believed to be a neural stem cell marker 8, but earlier studies have shown its expression in the epithelium of urinary bladder 9. C-kit is a hematopoietic stem cell (HSC) marker but is also expressed in the interstitial cells of Cajal (ICCs) of gastrointestinal and urinary tracts 10. In the present study we tested whether these markers could help identify potential stem cells in the urinary bladder.
Due to the lack of specific markers, potential epithelial stem cells in the urinary bladder have been tentatively identified by the “label-retaining cell (LRC)” strategy 11. In this study the authors intraperitoneally injected thymidine analog, 5-bromo-2-deoxyuridine (BrdU), into 6-week-old rats daily for 4 consecutive days. They then examined the presence of BrdU-positive cells in the bladder at intervals until 12 months post BrdU injection. However, the use of adult rats differs from the original and prevailing LRC protocol that calls for the use of newborn animals 12-14. In addition, the immunohistochemical detection of BrdU-labeled cells is difficult due to the subtle color difference between BrdU and nuclear stains. More importantly, the use of strong acids and high temperature in the detection procedure degrades cellular proteins, rendering them unrecognizable by their cognate antibodies. Consequently, determination of stem cell marker expression in BrdU-labeled cells is often not possible. To overcome these difficulties, we recently introduced a new stem cell labeling and detection method in which BrdU was replaced with 5-ethynyl-2-deoxyuridine (EdU) 15. The detection of EdU is a simple chemical reaction that requires no additional treatment of the tissue specimens, and the resulting signals are unambiguous. As such, we wished to test whether this new DNA label can help identify potential stem cells in the urinary bladder, in particular when combined with the above-mentioned stem cell markers and when assessed in developing, rather than adult, animals.
MATERIALS AND METHODS
Animals
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee at our institution. Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) for the investigation of childbirth-related urinary incontinence in separate projects. A total of 15 male neonatal pups delivered by these primiparous rats were used for this study. Each pup received intraperitoneal injection of EdU (50 mg/kg, Invitrogen, Carlsbad, CA) immediately after birth. Three rats were sacrificed at each of five time points (1d, 3d, 1w, 2w, 8w post-injection) for bladder harvest.
Preparation of tissue sections
Freshly dissected bladder tissue was fixed for 4 hours with cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer, followed by overnight immersion in buffer solution containing 30% sucrose. Tissues were frozen in optimum cutting temperature compound (Sakura Finetek, Torrance, CA), and stored at −80°C until use. Sections were cut at 5 μm, adhered to charged slides, and air dried for 5 minutes before staining.
Immunofluorescence staining
Frozen tissue sections were placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min, and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining solution from the tissue section, the tissue was incubated with rabbit anti-CD34 (sc-9095, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-SSEA-1 (MAB4301, Millipore Corporation, Billerica, MA), or mouse anti-smooth muscle actin (SMA) (099K4816, Sigma-Aldrich, St. Louis, MO), goat anti-Lgr5 (sc-68580, Santa Cruz Biotechnology), rabbit anti-c-Kit (sc-168, Santa Cruz Biotechnology). After rinses with PBS, the sections were incubated with FITC- or Texas Red-conjugated secondary antibody (Vector Laboratories, Burlingame, CA). Nuclear staining was performed with 4’,6-diamidino-2-phenylindole (DAPI). For tracking EdU-positive cells, tissue sections were incubated with Click-IT reaction cocktail (Invitrogen) for 30 min at room temperature.
Image and statistical analysis
Tissue slides were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments, Inc., Melville, NY). The images were then quantified using Image-Pro Plus image software (Media Cybernetics, Silver Spring, MD). For statistical analysis, three to five randomly selected fields per slides were examined and analyzed with Prism 5 (GraphPad Software, Inc., San Diego, CA). Data were expressed as mean ± standard deviation. One-way ANOVA was used to determine significance (p<0.05).
RESULTS
EdU labeling and retaining in the developing bladder
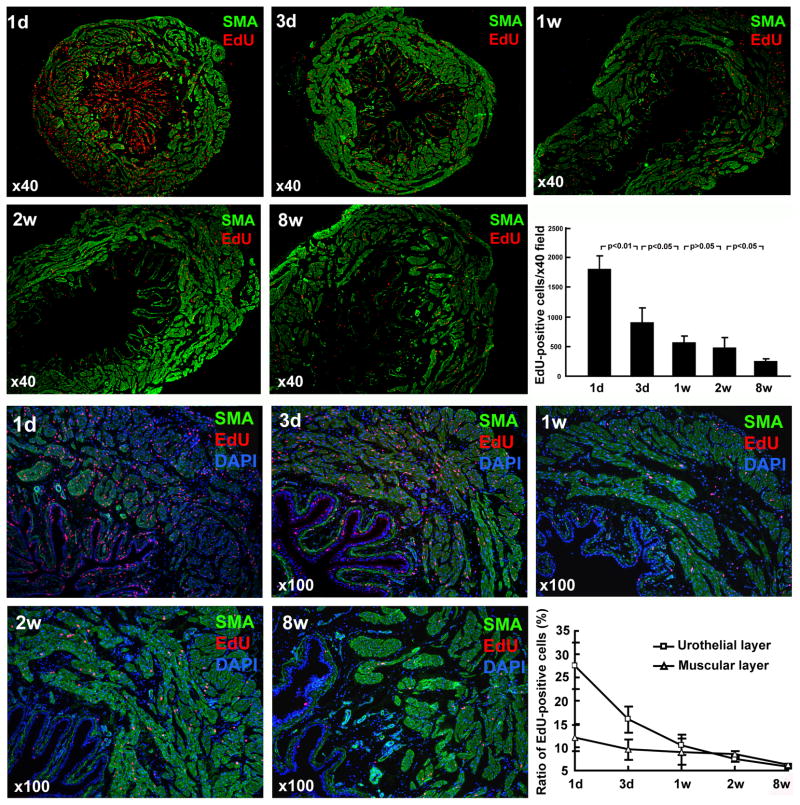
Intraperitoneal injection of EdU into newborn rats resulted in a high rate of bladder cell labeling (Fig. 1). At day one post-EdU injection, 1804.0±227.7 bladder cells were labeled in each cross section. Progressively fewer EdU-positive cells were present in the bladders of rats that were sacrificed at longer time points. Most labeling occurred in the epithelium with 27.5±4.9% of the epithelial cells getting labeled. Both the absolute and relative numbers of cells that remained labeled decreased with time. At 8 weeks, 236.5±53.0 cells per field at 40x magnification or 5.4±0.3% of epithelial cells remained labeled. In the detrusor, labeling rate was 12.1±2.8% one day after EdU injection and dropped to 5.6±0.3% at 8 weeks.
Figure 1.
EdU labeling and retaining in the developing bladder. Newborn rats were subject to intraperitoneal injection of EdU. Their urinary bladders were harvested at 1 day, 3 days, 1 week, 2 weeks, and 8 weeks later and processed for staining with SMA, EdU, and DAPI. Representative histological images at 40x and 100x are shown in the top and bottom two rows, respectively. The numbers of EdU-positive cells were counted at 40x magnification and presented in the bar chart. The ratios of EdU-positive cells (red stains) versus all cells (blue DAPI stain) were determined from the 100x photographs and presented in the line chart.
Stem cell marker expression in the mucosa
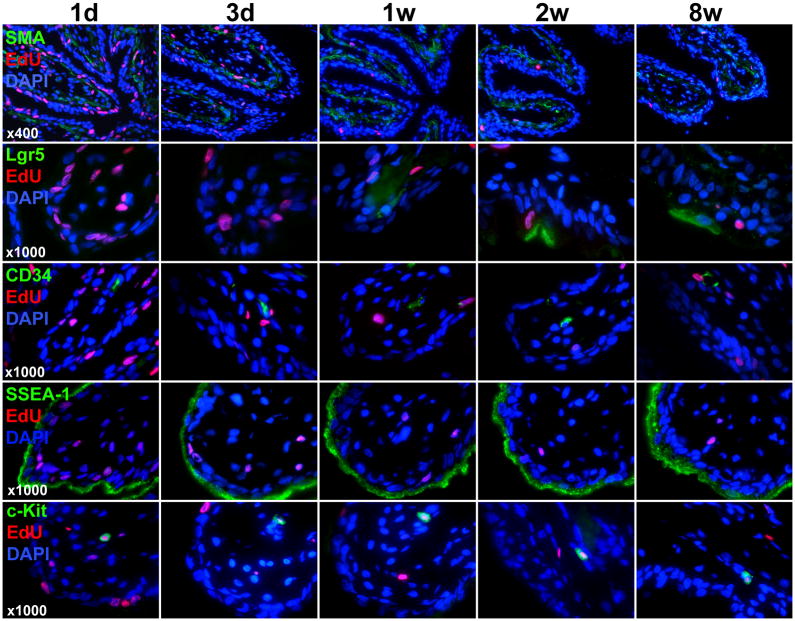
At day one post-EdU injection, the label was detectable in the urothelium and lamina propria, which can be seen as separated by the SMA-stained muscularis mucosae (Fig. 2). The number of EdU-labeled cells decreased sharply in both tissue compartments between the 1-day and 3-day samples. This trend of decreasing number of EdU-labeled cells and appearance of label-retaining cells in the mucosa continued in the 1-week, 2-week, and 8-week samples.
Figure 2.
Stem cell marker expression and EdU colocalization in the mucosa. Newborn rats were subject to intraperitoneal injection of EdU. Their urinary bladders were harvested at 1 day, 3 days, 1 week, 2 weeks, and 8 weeks later and processed for staining with SMA, EdU, and DAPI as well as the indicated stem cell marker (Lgr5, CD34, SSEA-1, or c-kit). Representative histological images of the mucosa at 400x and 1000x are shown for SMA (to row) and stem cell markers (bottom 4 rows), respectively.
Lgr5, an epithelial stem cell marker of the gastrointestinal tract, was not detectable in the bladder of 1-day- and 3-day-old rats, but was detectable albeit scantly in the bladder epithelium of 1-week-, 2-week-, and 8-week-old rats (Fig. 2). Some of the expressing cells were EdU-positive. CD34, a marker of endothelial cells and mesenchymal stem cells, was scantly detectable in the lamina propria; a few of these expressing cells were EdU-positive. Another stem cell marker, SSEA-1, was strongly expressed in the superficial layer (umbrella cells) of the urothelium; no colocalization with EdU was found.
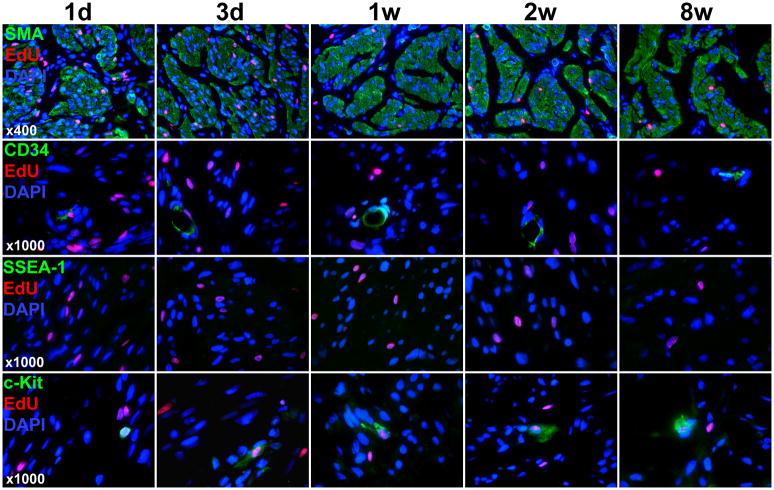
Stem cell marker expression in the detrusor
At day one Post-EdU injection, the label was detectable in the detrusor muscle and the interstitia (Fig. 3). As the rats grew older (1w, 2w, and 8w), the interstitia became having fewer cells (fewer DAPI stains); thus, the label was seen mostly in the detrusor muscle and rarely in the interstitia in these rats. CD34 expression was found to be associated with blood vessels with some degree of colocalization with EdU. SSEA-1 was undetectable.
Figure 3.
Stem cell marker expression and EdU colocalization in the detrusor. Newborn rats were subject to intraperitoneal injection of EdU. Their urinary bladders were harvested at 1 day, 3 days, 1 week, 2 weeks, and 8 weeks later and processed for staining with SMA, EdU, and DAPI as well as the indicated stem cell marker (CD34, SSEA-1, or c-kit). Representative histological images of the detrusor at 400x and 1000x are shown for SMA (to row) and stem cell markers (bottom 3 rows), respectively.
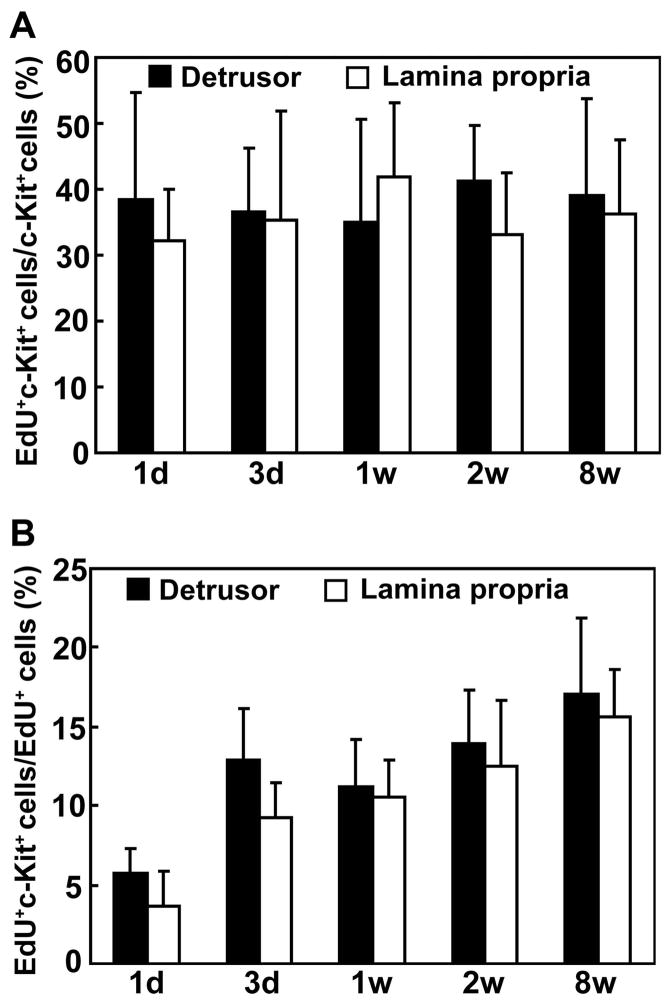
High rate of label retaining in c-kit-expressing cells
HSC marker c-kit is also a marker for ICCs and mast cells in the urinary bladder. In the present study c-kit was detected in the lamina propria and the detrusor (Fig. 2 & 3), locations known to harbor ICCs and mast cells. In both of these two tissue compartments the percentage of EdU-positive cells among c-kit-positive cells was largely constant at between 30 and 40 at any given time points after EdU injection (Fig. 4A). On the other hand, the percentage of c-kit-positive cells among EdU-positive cells increased sharply from 6 to 13 in the detrusor and 3 to 9 in the lamina propria between the 1-day and 3-day tissue samples (Fig. 4B). Thereafter, the rates stabilized. The co-localization of EdU and c-kit was slightly but consistently higher in the detrusor than in the mucosa.
Figure 4.
Co-localization rates for c-kit and EdU in the detrusor and lamina propria. Newborn rats were subject to intraperitoneal injection of EdU. Their urinary bladders were harvested at 1 day, 3 days, 1 week, 2 weeks, and 8 weeks later and processed for staining with c-kit and EdU. The percentages of cells positive for both EdU and c-kit among EdU-positive cells and among c-kit-positive cells are shown on the left and right panels, respectively.
COMMENT
Attempts to identify tissue-specific stem cells by the LRC strategy have been made on every major organ, often more than once. For the urinary bladder, only one such study exists, and the conclusion is that 9% of the urothelial basal cells retained the BrdU label 1 year after its administration 11. However, scoring BrdU-LRC is often guesswork as the indicative brown color is very difficult to tell apart from the concomitant purplish-brownish nuclear stain. As such, the present study wished to reassess the urinary bladder’s LRC profile by employing the EdU label whose detection is completely devoid of ambiguity 16–19. Also different from the previous study were the use of neonatal rats rather than adult rats, and a maximal time frame of 8 weeks rather than 1 year. Both of these two choices are consistent with most LRC studies. In regard to histology, we examined both the mucosa and detrusor while the previous study focused on the urothelium. In addition, we stained for stem cell markers Lgr5, CD34, SSEA-1, and c-kit while the previous study Bcl, p63, cytokeratin 14, and beta1 integrin.
In the present study, labeling of neonatal rat bladder by EdU occurred at a high rate, but the number of labeled cells dropped sharply within 2 days. These observations are consistent with most LRC studies and generally reflect the rapid cell cycling in most tissues of neonatal animals 12–14. Although initially (day 1) the urothelium had more than twice the labeling rate as the detrusor, these two compartments eventually (at 8 weeks) had similar rate of LRC. This epithelium versus detrusor difference mimics the epithelium versus stroma difference in the endometrium 14. Also noteworthy is that the distribution of the labeled cells was mostly random, whether in the urothelium or detrusor, and in all bladder tissues harvested at different time points after EdU injection. Thus, the results differ from the previous study, in which preferential labeling of urothelial basal cells was noted 11.
To examine stem cell marker expression in the bladder, particularly in LRC, four well-characterized markers that relate to epithelial, mesenchymal, hematopoietic, and neural stem cells were investigated. Epithelial stem cell marker Lgr5 was scantly detected in the urothelium one to eight weeks after EdU labeling, and some of the expressing cells were EdU-positive. But, there was no correlation between Lgr5 expression and label retaining. Mesenchymal stem cell marker CD34 was detected in the lamina propria and detrusor, mostly in association with blood vessels, at all time points after EdU labeling. While some CD34-expressing cells were EdU-positive, there was no correlation between CD34 expression and label retaining. Neural stem cell marker SSEA-1 was strongly expressed in the superficial layer (umbrella cells) of the urothelium. While this is in agreement with previous studies 9, no colocalization of SSEA-1 and EdU was found at any time points after EdU labeling.
The expression of HSC marker c-kit in the urinary bladder has been intensely studied. While it is expressed in both the mast cells and the ICCs, most attention has been given to the latter as these cells are believed to be the pacemaker of detrusor contraction 20. In the present study we detected c-kit expression in the lamina propria and the detrusor as previous studies did. More importantly, we found a high rate of co-localization in c-kit expression and EdU labeling. Specifically, approximately 30–40% of c-kit-expressing cells in either the lamina propria or the detrusor were labeled with EdU at any time points after EdU labeling (Fig. 4A). Thus, it appears that c-kit-expressing cells are proliferative in neonatal rat bladder (so as to get labeled) but soon become quiescent in the more matured bladder (so as to retain the label). This property of rapid cycling from proliferation to quiescence appears to be preferentially associated with c-kit expression, as the number of c-kit-expressing cells among EdU-positive cells increased sharply between day 1 and day 3 after EdU labeling and stabilized thereafter (Fig. 4B). While the significance of these findings is presently unknown, it should be pointed out that, in bone marrow, c-kit-expressing HSCs have been found to cycle rapidly from proliferative to quiescent state 21, and the function of c-kit has been shown to regulate the maintenance of quiescent HSC 22. In addition, c-kit expression has been associated with quiescent hepatic satellite cells 23, and ICCs in adult small intestine have been found to lack proliferative activity 24. Thus, the c-kit-expressing LRCs in the urinary bladder are probably quiescent ICCs.
Due to the rarity of tissue-specific stem cells and lack of specific markers, their identification by histological means is usually a first step and should be followed with more definitive approaches such as clonogenicity and plasticity tests. In the study by Kurzrock et al 11, a clonogenicity test was attempted with the assumption that a single BrdU-labeled cell isolated from rat bladder could give rise to a BrdU-labeled colony in culture. However, since a BrdU-labeled cell loses half of the label with each cell division, it is unlikely that all of the cells in a colony could be BrdU-positive as shown in the graph. Thus, the existence of bladder stem cells remains unsubstantiated by published studies or by the present study.
CONCLUSIONS
In regard to the urothelium and detrusor, labeling of bladder cells by EdU occurred randomly, and label retaining was not associated with expression of Lgr5, CD34, or SSEA-1. However, label retaining was strongly related to expression of c-kit, suggesting the existence of quiescent ICCs.
Acknowledgments
This work was supported by grants from the Rock Foundation, and the National Institutes of Health (DK045370, DK64538 and DK069655).
Glossary
- BrdU
5-bromo-2-deoxyuridine
- C-kit
CD117, also called KIT or c-kit receptor
- CD34
Cluster of Differentiation molecule 34
- DAPI
4’,6-diamidino-2-phenylindole
- EdU
5-ethynyl-2-deoxyuridine
- HSCs
Hematopoietic stem cells
- ICCs
interstitial cells of Cajal
- Lgr5
Leucine-rich G protein-coupled receptor 5
- LRCs
Label-retaining cells
- MSCs
Mesenchymal stem cells
- SMA
Smooth muscle actin
- SSEA-1
Stage-specific embryonic antigen-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol. 2011;193:257–266. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, Gibson P, Currle DS, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 6.Jaks V, Barker N, Kasper M, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 7.Lin CS, Xin ZC, Deng CH, et al. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 8.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 9.Parham DM, Morton K, Coghill G, et al. Expression of CD15 antigen in urinary bladder transitional cell carcinoma. J Clin Pathol. 1990;43:541–543. doi: 10.1136/jcp.43.7.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037–1051. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 11.Kurzrock EA, Lieu DK, Degraffenried LA, et al. Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol. 2008;294:F1415–1421. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- 12.Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998;244:184–195. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- 13.Duvillie B, Attali M, Aiello V, et al. Label-retaining cells in the rat pancreas: location and differentiation potential in vitro. Diabetes. 2003;52:2035–2042. doi: 10.2337/diabetes.52.8.2035. [DOI] [PubMed] [Google Scholar]
- 14.Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 15.Lin G, Huang YC, Shindel AW, et al. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy. 2009;11:864–873. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albersen M, Lin G, Fandel TM, et al. Functional, Metabolic, and Morphologic Characteristics of a Novel Rat Model of Type 2 Diabetes-associated Erectile Dysfunction. Urology. 2011 doi: 10.1016/j.urology.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YC, Shindel AW, Ning H, et al. Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol. 2010;183:1232–1240. doi: 10.1016/j.juro.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin G, Qiu X, Fandel T, et al. Tracking intracavernously injected adipose-derived stem cells to bone marrow. Int J Impot Res. 2011 doi: 10.1038/ijir.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin G, Wang G, Banie L, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011:233–254. doi: 10.1007/978-3-642-16499-6_11. [DOI] [PubMed] [Google Scholar]
- 21.Venezia TA, Merchant AA, Ramos CA, et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2:1640–1651. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thoren LA, Liuba K, Bryder D, et al. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180:2045–2053. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- 23.Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006;45:401–409. doi: 10.1016/j.jhep.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Mei F, Han J, Huang Y, et al. Plasticity of interstitial cells of cajal: a study in the small intestine of adult Guinea pigs. Anat Rec (Hoboken) 2009;292:985–993. doi: 10.1002/ar.20928. [DOI] [PubMed] [Google Scholar]