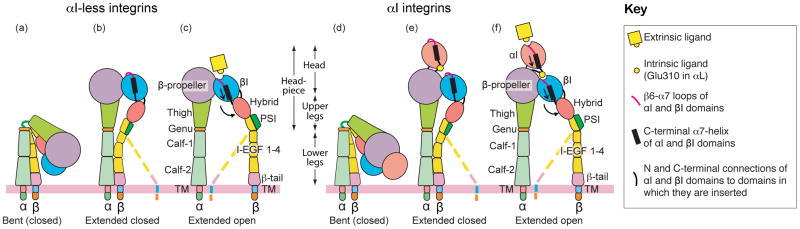
Figure 1.
The three overall integrin conformational states. The bent conformation has a closed headpiece and is low affinity. Extension at the α- and β-knees releases an interface between the headpiece and lower legs and yields an extended-closed conformation also with low affinity. Swing-out of the hybrid domain at its interface with the βI domain is connected through the βI α7-helix to rearrangements at the βI interface with the β-propeller domain that greatly (~1,000-fold) increase affinity for ligand in the extended-open conformation. Similar interdomain rearrangements in αI integrins result in activating a binding site for an internal ligand, Glu310 in αL, which pulls down the αI α7-helix to activate a similar increase in affinity (~1,000 to 10,000-fold) of the αL I domain for the ligand ICAM-1. Although the integrin headpiece has highly preferred closed and open conformations, the lower β-legs are highly flexible, and thus we speak of “overall” conformational states. This is symbolized by the dashed lower β-leg. Therefore, only very large separations between α and β TMD, such as induced by lateral motion of β when its cytoplasmic domain is associated with the actin cytoskeleton, can be transmitted through the floppy β-leg to stabilize the high-affinity, open headpiece conformation.