Figure 1. Outline of Study Design.
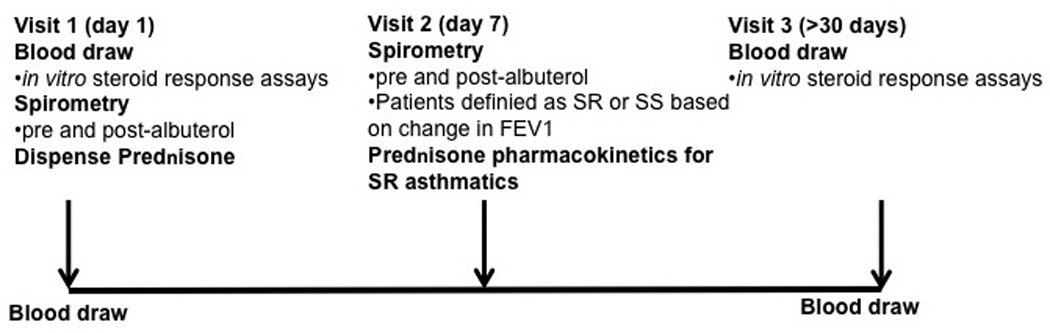
Asthmatics with a FEV1 % predicted <80% were recruited into the study. Blood was drawn at the time of initial oral prednisone administration (Visit 1) and 30 days post oral prednisone treatment (Visit 3). The patients returned 7 days post oral prednisone treatment for a spirometry testing (Visit 2). At this visit, patients were defined as SR if less then 10% improvement in FEV1 % predicted occurred, and as SS if >12% improvement in lung volume occurred. Glucocorticoid pharmacokinetics screen was done for all SR asthma patients. Only subjects with normal prednisone pharmacokinetics profile were included in the study.
