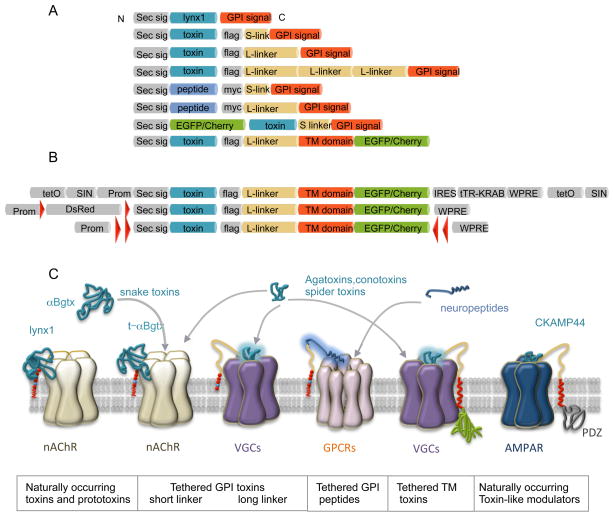
Figure 1. Scheme of the architecture and mode of binding of lynx1, derived engineered tethered toxins and neuropeptides and other naturally occurring toxin-like modulators.
(A) The tethered-peptide strategy uses the biological scaffold of lynx1 (cyan blue) to incorporate a secretory pathway signal sequence secretory signal (Sec sig: grey) and a GPI signal (red) to generate recombinant membrane-bound toxins and peptide ligands. Additional tethered-toxin and t-peptide variants consist of the secretory signal sequence (grey), toxin (cyan blue) or peptide (dark blue) ligand cassettes, fluorescence markers (EGFP or mCherry; in green), epitopes for immunostaining (flag-tag, myc-tag; in grey), flexible linker regions (beige), and distinct functional modules for membrane attachment (GPI signal or transmembranedomain TM; in red).
(B) Schematic of inducible and Cre-dependent viral vectors encoding t-toxins used for stereotactic injections in mice. Doxycycline inducible lentiviral vector containing tetO and tTR-KRAB cassettes [20]. Cre-dependent t-toxin viral vectors containing either loxP sites (red triangles) flanking DsRed [20], or a flip-excision (FLEX) switch with double loxP sites flanking the t-toxin. Prom: Promoter, WPRE: woodchuck post-transcriptional regulatory element.
(C) Illustration of lynx1 and GPI-tethered t-αBgtx binding to the nicotinic receptor (nAChR). Other peptide venom toxins, including agatoxins, conotoxins and spider toxins, and neuropeptides can be tethered to the membrane via a GPI anchor to cell specifically inactivate or modulate nAChRs, voltage gated channels (VGC) or G-protein couple receptors (GPCRs) depending on the selectivity of the t-toxin or t-peptide. T-toxins are also functional when tethered via a transmembrane (TM) domain. Curiously, CKAMP44, a naturally occurring toxin-like modulator of AMPAR [12] resembles TM t-toxins.