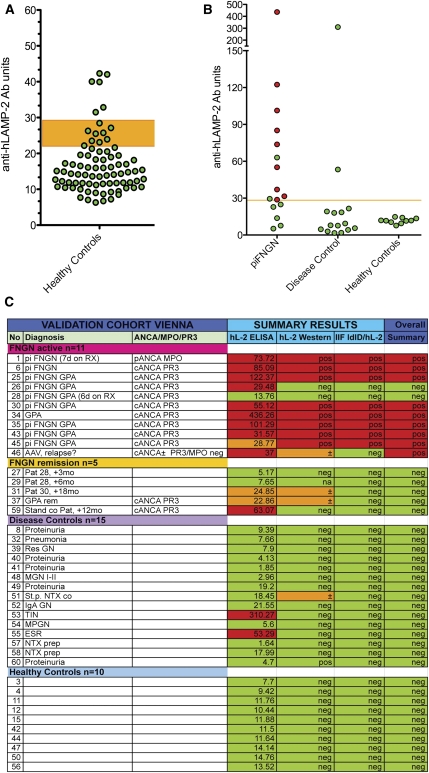
Figure 4.
Evaluation of assays for antibodies to LAMP-2. (A) ELISA results from a panel of sera from 78 healthy controls were used to derive the mean ± SD for this group and the 95% confidence limit of the upper limit of normal for the assay, which was established at 29 U. Six controls had positive ELISA with negative Western blots and IIF assays. (B) ELISA results of the data shown in A. The upper limit of normal in the assay is 29 U. Sera confirmed to have anti-hLAMP-2 antibodies by the other two assays are in red, whereas those in which positive ELISA was not confirmed because ELISA and IIF assays were negative are in green. (C) Measurement of antibodies to hLAMP-2 by ELISA and Western blot using hLAMP-2/GST FP and IIF on ldlD/hLAMP-2H cells was compared using a panel of 41 sera selected to cover a range of positive and negative values. The panel consisted of 16 sera from patients with AAV (11 with active disease and 5 in remission), 15 controls with other renal diseases, and 10 healthy controls. The assays were graded positive (red), low positive (Western and IIF), borderline (ELISA) (orange), or negative (green). The figure illustrates the strong concordance among results from the three assays. Sera were considered to have antibodies to LAMP-2 when ≥2 assays were positive. Abbreviations: TX, renal transplant; MGN, membranous nephropathy; IgA GN, IgA nephropathy; MPGN, membranoproliferative GN; TIN, tubulointerstitial nephritis.