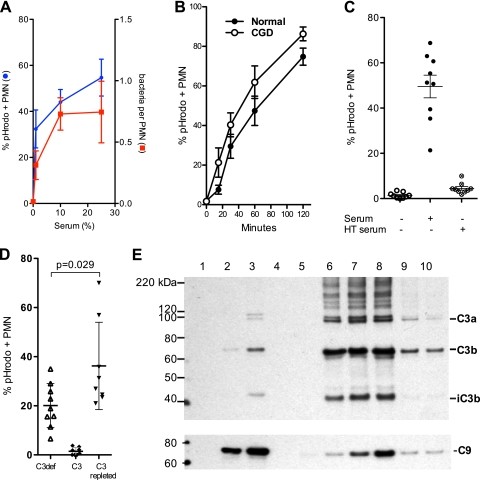
Fig 2.
G. bethesdensis is internalized by human PMN in a time- and serum-dependent manner. (A) Internalization of pHrodo-labeled bacteria was assessed microscopically by blinded scorers at 30 min and an MOI of 1:1. The percentage of all cells that contained at least 1 G. bethesdensis organism is plotted as a function of the serum dose (mean ± SEM; n = 9 healthy donors over 6 separate experiments) and the number of bacteria per PMN (mean ± SEM; n = 4 to 6 subjects). (B) Flow cytometry studies demonstrate internalization by normal or CGD PMN incubated with G. bethesdensis at an MOI of 10:1 in the presence of 10% autologous serum (mean ± SEM; n = 8 healthy patients and 4 patients with CGD). (C) Internalization of G. bethesdensis was assessed microscopically in the absence of serum or in the presence of either 10% serum or 10% heat-treated (HT; 56°C, 30 min) serum (mean ± SEM; n = 9). (D) Internalization of G. bethesdensis was measured microscopically in the presence of 10% C3-depleted serum alone, purified C3 at a concentration of 10 μg/ml, and both together (mean ± SEM of 9 healthy donors). Repletion of C3-depleted serum with C3 resulted in a significant increase in internalization (paired t test, 2 tailed). (E) Immunodetection of C3 and C9 bound to G. bethesdensis. Lane 1, bacteria alone; lane 2, 10% C3-deficient serum; lane 3, 10% C3-deficient serum plus 1 μg C3/ml; lane 4, bacteria alone; lane 5, 10% serum at 0 min; lane 6, 10% serum at 5 min; lane 7, 10% serum at 10 min; lane 8, 10% serum at 30 min; lane 9, 10% serum with EGTA plus MgCl2; lane 10, 10% serum previously heat treated at 50°C for 20 min. Blots are representative of at least 3 different binding experiments.