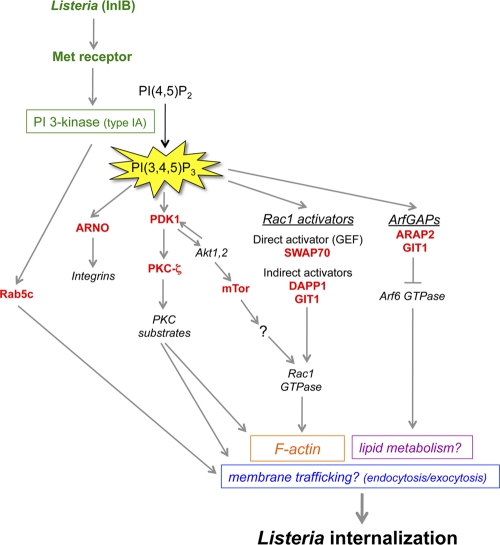
Fig 7.
Potential mechanisms of control of Listeria entry by the type IA PI 3-kinase signaling pathway. Infection of human cells with Listeria expressing InlB results in activation of the host Met receptor and of type IA PI 3-kinase (24, 45, 46, 78). The RNAi-based screen described in this work led to the identification of nine human proteins involved in PI 3-kinase signaling that play important roles in Listeria entry. Based on the biological functions of these nine proteins reported in the scientific literature, a diagram was constructed depicting some of the possible ways in which the host proteins could participate in bacterial uptake. Rab5c, a protein that interacts with regulatory and catalytic subunits of type IA PI 3-kinase, could promote Listeria entry by controlling the host endocytic machinery (14, 85). ARNO, an activator of Arf GTPases that binds directly to the PI 3-kinase product PI(3,4,5)P3, might help maintain proper levels of integrins, a class of receptor recently found to enhance InlB-mediated entry, in the plasma membrane (2). The serine/threonine kinase PKC-ζ could promote Listeria internalization by controlling the actin cytoskeleton and/or the delivery of membrane through exocytosis (7, 53, 73). PKC-ζ is indirectly regulated by PI 3-kinase through the master kinase PDK1, which is a direct target of PI(3,4,5)P3 (3). mTor, a serine/threonine kinase indirectly controlled by type IA PI 3-kinase, might promote bacterial uptake through activation of the host proteins Rac1 and/or PKC-α (not shown) (75, 91). Apart from mTor, three other human proteins identified in the RNAi screen have the potential to control Listeria entry though activation of Rac1 GTPase. These proteins, SWAP70, DAPP1, and GIT1, each bind directly to PI(3,4,5)P3. SWAP70 is a direct activator of Rac1 and stimulates nucleotide exchange on the GTPase (79). DAPP1 and GIT1 lack recognizable guanine nucleotide exchange factor (GEF) domains and likely activate Rac1 indirectly. In addition to being an indirect activator of Rac1, GIT1 is also a GTPase-activating protein (GAP) that inhibits Arf6 GTPase (41). Along with ARAP2, another Arf6 GAP needed for Listeria entry (34), GIT1 might restrain the activation of Arf6, which would otherwise interfere with bacterial uptake. Constitutively activated Arf6 alleles inhibit Listeria internalization (34) and also induce the redistribution of cholesterol from the plasma membrane to internal membrane compartments (65). Since plasma membrane cholesterol is critical for InlB-mediated entry, it is possible that GIT1 and/or ARAP2 promotes Listeria uptake by maintaining proper localization of cholesterol and/or other lipids (34).