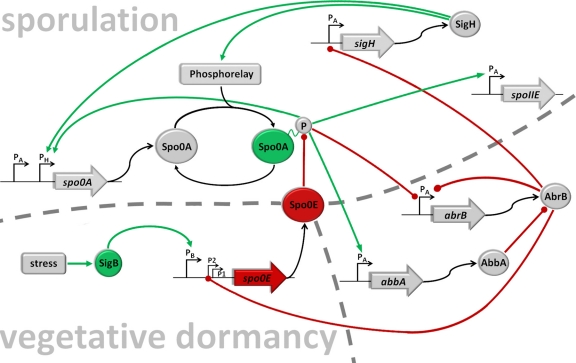
Fig 4.
The regulatory network. Shown is a simplified schematic representation of the core components relevant for the integration of the σB input into the regulatory network that governs initiation of sporulation. Lines ending with a dot (red) represent negative actions, and arrows (green) represent positive actions. To be active, Spo0A needs to be phosphorylated by a multicomponent phosphorelay (7) that integrates positive signals (27) and negative signals (18, 35). The spo0A gene is transcribed from a vegetative σA promoter and a sporulation-specific σH promoter (9, 40). Low levels of Spo0A∼P set in motion two parallel pathways of repression and antirepression that relieve AbrB-mediated repression of sigH (54), by directly repressing transcription of the abrB gene (15, 37, 53) and the induction of abbA encoding an antirepressor of the AbrB protein (3, 32). Derepression of sigH encoding the alternative sigma factor σH results in a positive autoregulatory loop by induction of the spo0A gene itself (15, 40) as well as the phosphorelay components KinA and Spo0F (15, 40). Furthermore, spo0A transcription from PσH is also under direct positive and negative control by Spo0A∼P in a concentration-dependent manner (56). Low levels of Spo0A∼P activate and high levels limit spo0A transcription to a constant upper or maximal level (15, 56). AbrB is a repressor for a wide variety of stationary-phase genes as well as its own synthesis (54). Inactivation of AbrB also causes a negative-feedback loop by derepression of spo0E from P2 (36) encoding a phosphatase Spo0E that directly removes phosphoryl groups from Spo0A∼P (33), converting it from an active to an inactive form; P1 of spo0E is constitutively active at low rates (36), and the third upstream promoter is recognized and induced by σB-containing RNA polymerase (this work). The spoIIE gene is directly activated by high threshold levels of Spo0A∼P from a σA-dependent promoter (10, 14) encoding a protein phosphatase that participates in σF activation.