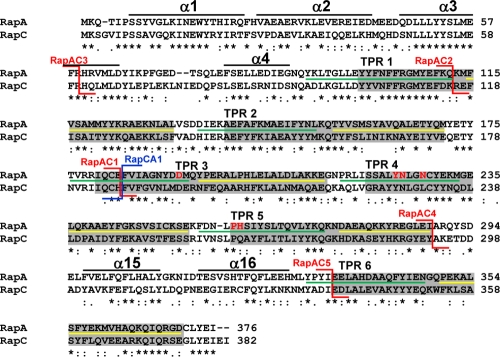
Fig 2.
Amino acid sequence alignment of RapA and RapC. The alignment was obtained with the ClustalW program. Asterisks indicate identical residues; colons and periods indicate conserved and semiconserved substitutions, respectively. The six TPR domains as defined by amino acid sequence conservation are in the gray boxes (31). The extent of the two α-helices that include each TPR domain, as determined by the crystal structure of RapH, is indicated by the green line (α1) or the yellow line (α2) (27). The position of the RapAC fusions is indicated by the red connectors, while the position of the RapCA fusion is shown by the blue connector. The residues corresponding to the regions in RapH that form four α-helices in the N terminus and the two α-helices in the connecting region between TPR5 and TPR6, identified by the crystal structure of the RapH-Spo0F complex, are shown by the black lines (27). The six residues affected in PhrA binding are shown in red (D192, Y224, N225, N228, H260, and P259).