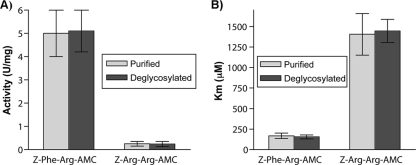
Fig 5.
Analysis of the activity of the nondeglycosylated and deglycosylated EtCatB produced in P. pastoris toward the Z-F-R-AMC and Z-R-R-AMC substrates. (A) Representation of the activity (U/mg protein) of the different enzyme fractions against the two substrates Z-F-R-AMC and Z-R-R-AMC. Enzyme samples were tested for activity in a final volume of 1 ml. The enzyme sample was incubated for 10 min at 37°C in the assay buffer before the reaction was started by addition of the substrate (200 μM; for the Km determinations, concentrations of 10 μM to 1.5 mM were used). Excitation and emission wavelengths were, respectively, 380 and 465 nm. The assays were continuous for 10 min at 37°C. (B) Representation of the Km values (μM) for the different enzyme fractions against the two substrates Z-F-R-AMC and Z-R-R-AMC.