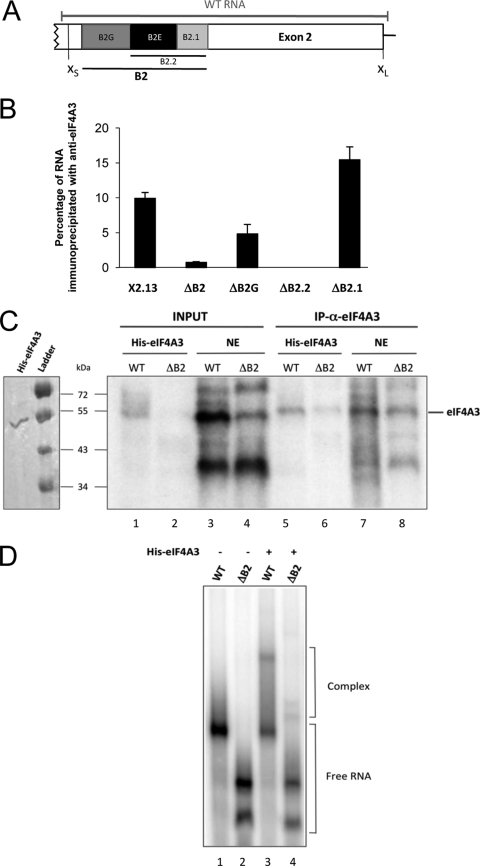
Fig 4.
eIF4A3 interacts directly with the Bcl-x pre-mRNA via the B2 element. (A) Diagram of the alternative exonic region located between the alternative Bcl-x 5′ splice sites. The B2 element is depicted with its subregions B2G, B2.2, and B2.1. The segment designated WT RNA represents the in vitro-transcribed RNA used for panels C and D. (B) Following the incubation of the various Bcl-x transcripts in a HeLa nuclear extract, labeled RNA was immunoprecipitated with anti-eIF4A3. The percentage of immunoprecipitated labeled RNA (relative to input and subtracted from that for a mock immunoprecipitation performed with each pre-mRNA) is plotted. (C) Cross-linking assays were performed in the presence of labeled WT or ΔB2 transcripts incubated for 30 min on ice with recombinant His-eIF4A3 or a HeLa nuclear extract, followed by UV irradiation and immunoprecipitation with anti-eIF4A3. Samples were fractionated on a 10% polyacrylamide-SDS gel. The left panel displays a Coomassie blue-stained gel of our purified His-eIF4A3 protein. (D) Labeled WT or ΔB2 transcripts were incubated for 25 min at 30°C in the presence (+) or the absence (−) of 1 μg recombinant protein His-eIF4A3. Complexes were separated on a native 4.5% acrylamide gel.