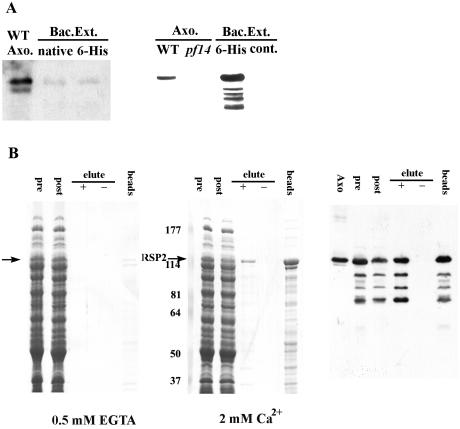
FIG. 6.
Recombinant RSP2 binds calmodulin in a Ca2+-dependent manner. (A) Extracts from bacteria transformed with the expression constructs for either native or six-His-tagged RSP2 were analyzed by Western blotting with monoclonal (left panel) and polyclonal (right panel) anti-RSP2 antibodies. Axonemes from wild-type and pf14 cells (lane 2, right panel) and the bacterial extracts without the fusion proteins (lane 4, right panel) served as controls. Compared to the polyclonal antibody (right panel), the monoclonal antibody (left panel) recognized the recombinant protein with significantly lower affinity. WT, wild type. (B) Calmodulin affinity chromatography was performed on extracts from the bacteria expressing native RSP2 in the presence (middle panel, Coomassie blue protein stain; right panel, parallel Western blot with RSP2 antibody) and absence (left panel) of calcium, and then the proteins were eluted from the calmodulin matrix with 2 mM EGTA (“+” symbol, all panels). In the presence of EGTA, no binding of RSP2 was detected (arrow, left panel). In contrast, when Ca2+ was present, RSP2 bound to the matrix (compare “pre” and “post” columns, middle and right panels), and in comparison, no bacterial proteins were visibly reduced following extraction with the calmodulin-Sepharose matrix. A fraction of the bound RSP2 can be eluted with EGTA (compare “−” and “+” lanes, middle and right panels), including degradation products of the expressed RSP2 revealed by Western blotting (right panel). However, a large fraction of the bound RSP2 remained associated with the calmodulin matrix following EGTA elution (“beads,” middle and right panels), indicating strong affinity between a fraction of RSP2 and calmodulin. Numbers at left of the middle panel are molecular masses in kilodaltons.