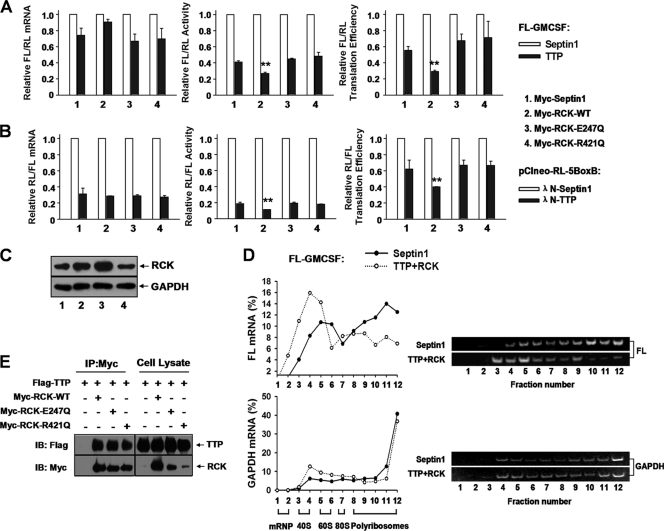
Fig 9.
RCK promotes TTP-mediated translation repression and is dependent on its helicase activity. (A) Wild-type RCK, but not its mutants RCKE247Q and RCKR421Q, acts together with TTP to repress FL-GM-CSF reporter translation. 293T cells were transfected with the FL-GM-CSF reporter and RL plasmids, together with two plasmids, one expressing either HA-tagged TTP or HA-septin 1 and another expressing either Myc-tagged RCK or its mutated forms, as indicated. The relative values of FL activity, mRNA level, and translation efficiency were set to 1 for cells transfected with the plasmid expressing HA-tagged septin 1 in each condition. Means and SD from three independent experiments are shown. **, P < 0.01. (B) Wild-type RCK promotes TTP-mediated translational repression in tethering assays, but its mutants RCKE247Q and RCKR421Q do not. 293T cells in 24-well plates were transfected with 40 ng of pCIneo-RL-5BoxB and 50 ng of FL-Control, together with two plasmids, one expressing either λN-TTP or septin 1 (10 ng/well) and another expressing either Myc-tagged RCK or its mutated forms (50 ng/well), as indicated. The relative values of RL activity, mRNA level, and translation efficiency were set to 1 for cells transfected with the λN-septin 1 plasmid in each condition. Means and SD from three independent experiments are shown. **, P < 0.01. (C) Expression levels of RCK were analyzed by Western blotting using anti-RCK antibody. GAPDH served as a loading control. Results show that transfection with plasmids expressing Myc-tagged RCK-WT (lane 2) or its mutants RCKE247Q (lane 3) and RCKR421Q (lane 4) increased RCK protein level about 2-fold compared with control septin 1 transfection (lane 1). (D) Accumulation of FL-GM-CSF reporter mRNA in polyribosomes is significantly reduced upon cotransfection of TTP and RCK. 293T cells in 100-mm dishes were transfected with 4 μg of FL-GM-CSF reporter, together with plasmids expressing HA-TTP (2 μg) and Myc-RCK (2 μg). The HA-septin 1 plasmid served as a control. Forty-eight hours later, cytoplasmic lysates were prepared and loaded onto 10-to-50% sucrose gradients as described for Fig. 1D. Representative data from three independent experiments are shown, with ribosome subunit distributions indicated below the bottom graph. (E) The helicase domain mutant of RCK does not affect its association with TTP. The indicated plasmids were transfected into 293T cells, and cytoplasmic lysates were immunoprecipitated with anti-Myc antibody immobilized on agarose beads. Inputs and immunoprecipitates were analyzed by Western blotting with anti-Myc or anti-Flag antibody.