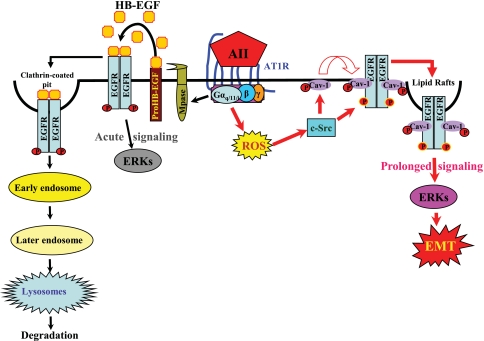
Fig 10.
A novel mechanism linking ROS/Src/caveolin-mediated activation of the EGFR-ERK signaling pathway to chronic Ang II exposure-induced EMT in cultured renal tubular epithelial cells. Our studies utilizing the renal proximal tubular epithelial cells expressing functioning AT1 receptors suggest that binding of Ang II to the seven-transmembrane G-protein-coupled AT1 receptor activates metalloproteinase (Mpase)-dependent release of soluble HB-EGF, a natural EGFR ligand that binds and transactivates EGFR, as we have recently documented (5), resulting in acute signaling leading to ERK activation; such ligand-activated ERK signaling diminishes within 1 h (Fig. 5, 6, and 8C). The transient nature of this EGFR-ERK signaling is presumably due to the quick endocytosis of the ligand (HB-EGF)-activated EGFR through clathrin-coated pits, followed by degradation through the endosomal/lysosomal pathway (9, 39). Meanwhile, Ang II activation of AT1 receptors also leads to transactivation of EGFR through ROS-dependent Src kinase activation, which phosphorylates both caveolin-1 (Cav) at Y14 and EGFR at Y845, thus inducing association of EGFR with Cav and the adaptor proteins SHC and GRB2, resulting in prolonged EGFR-ERK signaling. In the case of chronic Ang II exposure, such prolonged signaling activity persists in the epithelial cells, leading to alterations in gene expression and consequently eliciting a phenotypic change to EMT.