Fig. 3.
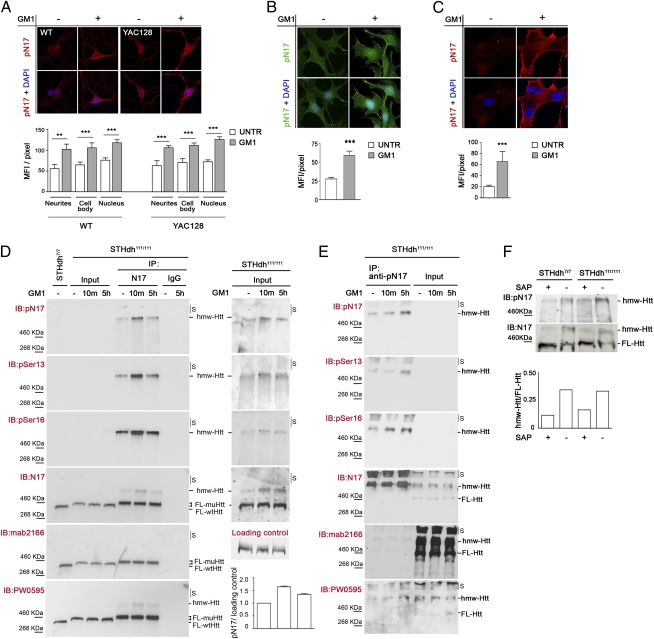
GM1 administration elicits huntingtin phosphorylation at serine 13 and serine 16. (A) Representative confocal microscopy images and relative quantitative analysis of primary striatal neurons isolated from wild-type (WT) and YAC128 mice and incubated for 5 h with 50 μM GM1 (+) or vehicle (−). Neurons were immunostained with anti–phospho-N17 (pN17) antibody, which recognizes the amino-terminal N17 peptide of huntingtin phosphorylated at amino acid residues serine 13 and serine 16, and with DAPI to visualize nuclei. (B) Representative epifluorescence microscope images and quantitative analysis of immortalized knock-in striatal progenitor cells expressing mutant huntingtin (STHdh111/111) and treated as in A. (C) Confocal microscopy images and quantitative analysis of primary fibroblasts from HD patients treated as in A. Graphs in A–C show pN17 immunostaining mean fluorescence intensity (MFI) per pixel ±SD, calculated over a minimum of 100 cells per experimental group. **P < 0.01; ***P < 0.001. (D and E) Analysis of mutant huntingtin phosphorylation state by immunoprecipitation and immunoblotting. Striatal knock-in cells (STHdh111/111) were incubated with 50 μM GM1 for the indicated time (10m, 10 min). Mutant huntingtin was immunoprecipitated from equal amounts of total cell lysates using a rabbit polyclonal anti-huntingtin antibody (N17) (in D), phospho-specific pN17 antibodies (in E), or nonspecific rabbit IgG antibodies as negative control (D). Total lysate from cells expressing wild-type huntingtin (STHdh7/7) was loaded in the same gel as reference. All immunoprecipitated material (IP) was immunoblotted with the indicated phospho-specific and anti-huntingtin antibodies. Phosphohuntingtin could not be detected in the total lysates (input lanes, 30 μg of proteins loaded) due to the proximity of the highly immune-reactive immunoprecipitated material in adjacent lanes. The results of reprobing the input lanes only, after cutting the membrane, are shown in D, Right. An increase in the phosphorylation of huntingtin at serine 13 and serine 16 after treatment with GM1 is evident in both immunoprecipitated material and input total cell lysates. The graph in D shows the densitometric analysis of huntingtin phosphorylation in the input lysates of two independent experiments. A Ponceau red-stained band in the membrane was used as loading control. S, stacking portion of the gel. (F) Wild-type and mutant huntingtin in total cell lysate from striatal knock-in cells (STHdh7/7 and STHdh111/111) was dephosphorylated using shrimp alkaline phosphatase (SAP). Dephosphorylation resulted in a dramatic change in huntingtin electrophoretic mobility, with increased amount of protein migrating at the lower apparent molecular weight (FL-Htt) and a decreased amount of high molecular-weight species (hmw-Htt). The graph shows the ratio between hmw-Htt and FL-Htt before and after dephosphorylation with SAP.