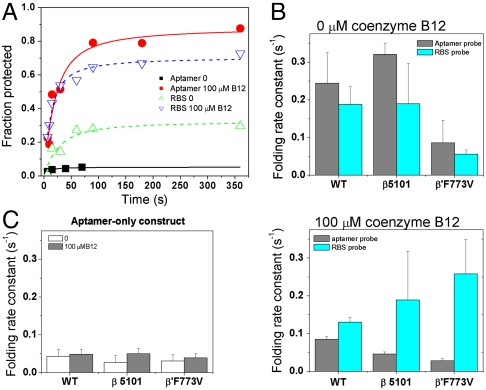
Fig. 4.
Pausing-deficient RNAP affects folding kinetics. The cleavage reaction with the RBS probe was carried out with 25% the amount of RNase H, as compared to the cleavage reaction with the aptamer probe. (A) Folding kinetics of the aptamer and expression platform during transcription by the wild-type RNAP. (B) The folding rate constants of the aptamer domain and the expression platform are within 1.5-fold to each other when transcribed by the wild-type enzyme, both in the absence and presence of coenzyme B12 ligand. In contrast, transcription by the pausing-deficient, fast RNAPs leads to increased divergence of the folding rates, especially in the presence of coenzyme B12. (C) Folding rates of the aptamer-only construct do not depend on the RNAP used in the transcription.