Bacteria commonly encode virulence determinants that mediate the disruption of host cell membranes; however, vacuole lysis and invasion of the host cell cytosol is a strategy used by only a select few bacterial pathogens for intracellular replication (1). Many bacterial pathogens replicate in specialized vacuoles that must be actively maintained to prevent these organelles from fusing with lysosomes and to regulate acquisition of new membrane for vacuole expansion during bacterial replication (2). However, why is this, when, in theory, it should be easier for a bacterium to lyse the vacuole and replicate in the cytosol? A study in PNAS provides new data that demonstrate that vacuole integrity is important for the intracellular survival of Legionella pneumophila and illustrate how invasion of the host cytosol can play a role in countering bacterial infection (3).
L. pneumophila is found commonly in fresh water environments and soil. When Legionella are engulfed by parasitic protozoan hosts, the bacteria rapidly use a type IV secretion apparatus called Dot/Icm to deliver a large number of effector proteins that promote intracellular survival and replication (4). Genomic analysis has revealed extensive plasticity in the repertoire of genes encoding these effectors among different species and serogroups of Legionella (5). It is thought that effector plasticity is driven in large part by the genetic diversity among the protozoan hosts that cohabitate the environments in nature where Legionella reside.
For humans, serious health problems can result if aerosols are generated from an environmental source containing a high density of Legionella. Bacteria entering the lung are internalized and replicate within resident alveolar macrophages. This infection triggers a robust innate immune response that is very effective at activating macrophages and recruiting neutrophils that are able to internalize and kill Legionella (6). When high numbers of Legionella are present in the lung, this robust innate immune response and the resulting pulmonary infiltrate can manifest as a severe pneumonia called Legionnaires disease.
Mouse macrophages are intrinsically more resistant to Legionella infection compared with human macrophages (7). This property has been exploited to identify bacterial factors that are sensed by macrophages to promote cell autonomous responses that restrict pathogen replication. The power of this system was illustrated when mouse macrophages were used to identify mutant Legionella that could escape host detection by displaying an enhanced intracellular replication phenotype (8, 9), which aided in the discovery of a cell death pathway in mouse macrophages that is activated when Legionella flagellin is sensed by the host receptor Naip5 (10, 11).
In PNAS, Creasey and Isberg show that the robust ability of Legionella to activate host pathways that restrict bacterial replication in macrophages can be leveraged to better understand the function of effector proteins delivered by the Dot/Icm system (3). This study focused on an effector called SdhA, which gained notoriety among the estimated 275 different effectors because it is one of the only proteins that, when eliminated from the effector repertoire, results in a defect in the intracellular replication of Legionella (12). Although a measurable phenotype is observed in protozoan hosts and in human macrophage-like cells, the intracellular replication defect displayed by a Δ;sdhA strain is extremely strong when using macrophages derived from an A/J strain of mouse that is unable to efficiently sense Legionella flagellin as a result of a defect in the gene encoding the Naip5 protein.
However, why would the Δ;sdhA phenotype be so severe in mouse macrophages? The authors found that infection of mouse macrophages with the Δ;sdhA mutant resulted in enhanced induction of many cytosolic immune surveillance pathways, including pathways that lead to cell death and type-I IFN production (3). Surprisingly, an examination of macrophages infected with Δ;sdhA mutants revealed vacuole instability that resulted in the appearance of Legionella in the host cytosol. Legionella in the cytosol were targeted for destruction, possibly by autophagy-directed trafficking of bacteria to degradative organelles. Attempts to eliminate individual host cytosolic surveillance pathways were insufficient to complement the Δ;sdhA replication defect in macrophages, indicating that cytosolic bacteria are potent agonists of multiple pathways that can independently restrict bacterial replication or perhaps the critical pathway induced by these cytosolic bacteria represents a new pathway.
To better understand the interesting phenotype displayed by the Δ;sdhA strain, macrophages were used to enrich for bacteria that could escape the robust growth restriction displayed by mammalian host cells, which led to the identification of suppressor mutations that resulted in enhanced intracellular replication of the Legionella Δ;sdhA strain. The strongest suppressors of the Δ;sdhA phenotype were loss-of-function mutations in the plaA gene, which eliminate production of a secreted phospholipase (13). The integrity of vacuoles containing the Δ;sdhA mutant was enhanced when the plaA gene was deleted or when the phospholipase activity of the enzyme was inactivated by site-directed mutation. Thus, the phospholipase activity of PlaA renders vacuoles more susceptible to disruption in the absence of SdhA (Fig. 1).
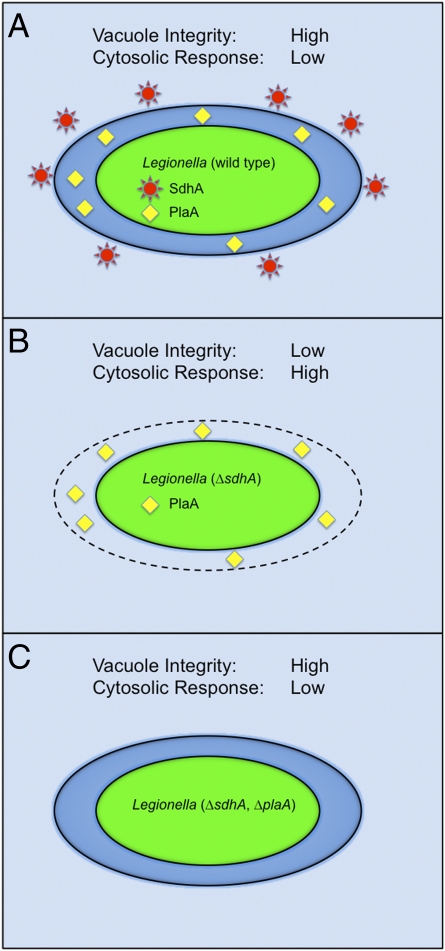
Fig. 1.
Properties displayed by vacuoles containing Legionella. (A) Vacuoles containing wild type Legionella deliver the SdhA protein into the cytosol and the PlaA protein into the vacuole lumen. These vacuoles remain intact and infected macrophages do not display enhanced activation of cytosolic surveillance pathways. (B) Vacuoles containing the Δ;sdhA mutant display a loss of membrane integrity and enhanced activation of cytosolic surveillance pathways is observed. (C) Vacuoles containing the Δ;sdhA, Δ;plaA double mutant are stable and macrophages do not show enhanced activation of cytosolic surveillance pathways.
The epistatic relationship between SdhA and PlaA in maintaining vacuole integrity is very similar to that reported for the effector proteins SifA and SseJ from the vacuolar pathogen Salmonella enterica serovar Typhimurium, in which vacuoles containing sifA mutant bacteria display integrity defects that can be suppressed by mutations in sseJ (14, 15). Interestingly, SseJ and PlaA are both lipases belonging to the GDSL family of proteins (16). Although there is no homology or predicted structural similarity, this raises the possibility that SifA and SdhA could perform similar functions. The SifA protein modulates membrane dynamics on vacuoles containing Salmonella and is required for the formation of tubules that emanate from the vacuole. In the absence of SifA, membrane dynamics are perturbed in a manner that enhances vacuole disruption, and SseJ function augments this effect. Thus, SdhA may also be involved in controlling membrane dynamics, and without SdhA function, the vacuole containing Legionella becomes less stable, in part because of the lipolytic activities of PlaA.
The question remains why the requirement for SdhA is stronger when using mouse macrophages compared with
Vacuole integrity is important for the intracellular survival of Legionella pneumophila.
human macrophages or protozoan hosts. Is vacuole disruption enhanced in mouse macrophages? This could indicate that PlaA activity in mouse macrophages is elevated compared with macrophages from other animals. Alternatively, paralogous effectors predicted to have biochemical activities similar to SdhA could function better in other model host cells compared with mouse macrophages (12). If vacuole disruption were similar in the different host cells, it could mean that mouse macrophages respond more robustly to cytosolic Legionella compared with other cells or that these cells have an additional cytosolic surveillance mechanism that is not expressed in more permissive cell types.
The concept that the cytosol of mammalian cells might not be a good growth medium for bacteria was suggested by studies in which different bacteria were microinjected directly into the cytosol of the epithelial cell line Caco-2, and it was found that a number of vacuolar pathogens were unable to replicate in the cytosol (17). Although the mechanisms by which the host cytosol can sense and restrict pathogen replication remain largely unexplored, it has been shown that bacteria in the cytosol can be recognized by the host ubiquitination machinery (18–20). Bacterial ubiquitination is a signal that promotes compartmentalization of cytosolic invaders into autophagosomes and promote degradation. These new studies with Legionella indicate that cytosolic invasion can also activate cell death pathways and suggest that there may be pathways not yet characterized that are important for cytosolic immunity to microbial invasion. Thus, Legionella may prove to be a valuable reagent to better understand the mechanisms mammalian cells have evolved to combat cytosolic invasion by bacteria.
Acknowledgments
Work on Legionella infection and immunity in the author's laboratory is supported by National Institutes of Health Research Grants R01 AI041699 and R01 AI048770.
Footnotes
The author declares no conflict of interest.
See companion article on page 3481.
References
- 1.Goebel W, Kuhn M. Bacterial replication in the host cell cytosol. Curr Opin Microbiol. 2000;3:49–53. doi: 10.1016/s1369-5274(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 2.Alix E, Mukherjee S, Roy CR. Subversion of membrane transport pathways by vacuolar pathogens. J Cell Biol. 2011;195:943–952. doi: 10.1083/jcb.201105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creasey EA, Isberg RR. The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proc Natl Acad Sci USA. 2012;109:3481–3486. doi: 10.1073/pnas.1121286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ensminger AW, Isberg RR. Legionella pneumophila Dot/Icm translocated substrates: A sum of parts. Curr Opin Microbiol. 2009;12:67–73. doi: 10.1016/j.mib.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Valero L, Rusniok C, Cazalet C, Buchrieser C. Comparative and functional genomics of legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front Microbiol. 2011;2:208. doi: 10.3389/fmicb.2011.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol. 2008;10:1209–1220. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S, Mizuguchi Y. Multiplication of Legionella pneumophila Philadelphia-1 in cultured peritoneal macrophages and its correlation to susceptibility of animals. Can J Microbiol. 1986;32:438–442. doi: 10.1139/m86-083. [DOI] [PubMed] [Google Scholar]
- 8.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamboni DS, et al. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 12.Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci USA. 2006;103:18745–18750. doi: 10.1073/pnas.0609012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flieger A, Neumeister B, Cianciotto NP. Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect Immun. 2002;70:6094–6106. doi: 10.1128/IAI.70.11.6094-6106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz-Albert J, et al. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol. 2002;44:645–661. doi: 10.1046/j.1365-2958.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 15.Beuzón CR, et al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akoh CC, Lee GC, Liaw YC, Huang TH, Shaw JF. GDSL family of serine esterases/lipases. Prog Lipid Res. 2004;43:534–552. doi: 10.1016/j.plipres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Goetz M, et al. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc Natl Acad Sci USA. 2001;98:12221–12226. doi: 10.1073/pnas.211106398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 2012 doi: 10.1038/nature10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amano A, Nakagawa I, Yoshimori T. Autophagy in innate immunity against intracellular bacteria. J Biochem. 2006;140:161–166. doi: 10.1093/jb/mvj162. [DOI] [PubMed] [Google Scholar]
- 20.Perrin AJ, Jiang X, Birmingham CL, So NS, Brumell JH. Recognition of bacteria in the cytosol of Mammalian cells by the ubiquitin system. Curr Biol. 2004;14:806–811. doi: 10.1016/j.cub.2004.04.033. [DOI] [PubMed] [Google Scholar]