Abstract
A metabolic study revealed that 28-norcastasterone in Arabidopsis is synthesized from cholesterol via the late C-6 oxidation pathway. On the other hand, the early C-6 oxidation pathway was found to be interrupted because cholestanol is converted to 6-oxocholestanol, but further metabolism to 28-norcathasterone was not observed. The 6-oxoBRs were found to have been produced from the respective 6-deoxoBRs administered to the enzyme solution, thus indicating that these 6-oxoBRs are supplied from the late C-6 oxidation pathway. Heterologously expressed CYP85A1 and CYP85A2 in yeast catalysed this C-6 oxidation, with CYP85A2 being much more efficient than CYP85A1. Abnormal growth of det2 and dwf4 was restored via the application of 28-norcastasterone and closer precursors. Furthermore, det2 and dwf4 could not convert cholesterol to cholestanol and cholestanol to 6-deoxo-28-norcathasterone, respectively. It is, therefore, most likely that the same enzyme system is operant in the synthesis of both 28-norcastasterone and castasterone. In the presence of S-adenosyl-L-methionine, the cell-free enzyme extract catalysed the C-24 methylation of 28-norcastasterone to castasterone, although the conversion rates of 28-norteasterone to teasterone and 28-nortyphasterol to typhasterol were much lower; this suggests that 28-norcastasterone is the primary precursor for the generation of C28-BRs from C27-BRs.
Keywords: Arabidopsis thaliana, brassinosteroids, C27-BRs biosynthesis, 28-norcastasterone
Introduction
The absence of brassinosteroids (BRs) in the Arabidopsis mutants det2, cpd, and dwf4 (Li et al., 1996; Szekeres et al., 1996; Choe et al., 1998; Noguchi et al., 1999), tomato dwarf (Bishop et al., 1999), and pea lkb (Nomura et al., 1997, 1999) results in pleiotropic abnormalities, including reduced shoot elongation, reduced fertility, delayed senescence, and altered vasculature and photomorphogenesis. Mutants can be restored to the wild-type phenotype via the application of BRs. Similar abnormalities are also observed in the Arabidopsis mutants bri1 (Li and Chory, 1997), bin2 (Li et al., 2001; Li and Nam, 2002), and bak1 (Nam and Li, 2002), as well as in the tomato mutant curl-3 (Koka et al., 2000). However, the mutant phenotype cannot be rescued by the application of BRs because of disrupted BR signalling. Therefore, BRs are currently regarded as essential plant hormones whose endogenous levels must be properly maintained in plant cells to facilitate normal growth and development.
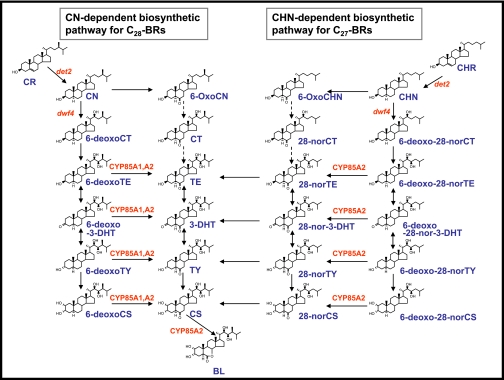
Naturally-occurring BRs, the number of which totals over 50, can be classified into C27-, C28-, or C29-BRs based on the nature of the alkyl groups occupying the C-24 position in the side chain of the 5α-cholestane carbon skeleton. Among them, the C28-BRs that harbour a C-24 methyl group are major BRs in the plant kingdom. Castasterone (CS) and brassinolide (BL) belonging to the C28-BRs are biologically highly active and, therefore, have been extensively investigated for their biosyntheses by means of feeding experiments as well as molecular genetics of BR-deficient mutants. According to the results, two parallel pathways—namely the early and late C-6-oxidation pathway in plant cells—have been proposed (Fujioka et al., 1997; Yokota, 1997; Sakurai, 1999; Bishop and Yokota, 2001; Fujioka and Yokota, 2003; Fig. 1). The biosynthesis of C28-BRs begins with the hydrogenation of campesterol to campestanol. In the early C-6 oxidation pathway, campestanol is then oxidized to 6-oxocampestanol, which undergoes successive oxidation to cathasterone (CT), teasterone (TE), 3-dehydroteasterone (3-DHT), typhasterol (TY), and CS. In the late C-6 oxidation pathway, campestanol is first oxidized at C-22 to generate 6-deoxocathasterone (6-deoxoCT), which is then oxidized successively to 6-deoxoteasterone (6-deoxoTE), 6-deoxo-3-dehydroteasterone (6-deoxo-3-DHT), 6-deoxotyphasterol (6-deoxoTY), 6-deoxocastasterone (6-deoxoCS), and CS. Finally, CS is oxidized to BL with a 7-oxalactone moiety.
Fig. 1.
Biosynthetic pathways for C27- and C28-BRs and their connection established in A. thaliana. The solid and dashed arrows indicate verified and not verified biosynthetic steps, respectively. The names on arrows indicate genes or enzymes catalysing biosynthetic reactions. (This figure is available in colour at JXB online.)
28-Norcastasterone (28-norCS), a C27 counterpart of CS, has also been identified from as many as 12 plant tissues, although less frequently than CS (Fujioka, 1999; Fujioka et al., 2000; Bajguz and Tretyn, 2003). 28-NorCS possesses the same carbon skeleton as cholesterol, thus suggesting that 28-norCS is synthesized from cholesterol in a fashion similar to the synthesis of CS from campesterol. Tomato seedlings were determined to contain cholesterol, cholestanol, and several 6-deoxo-28-norBRs including 6-deoxo-28-norcathasterone (6-deoxo-28-norCT), 6-deoxo-28-nortyphasterol (6-deoxo-28-norTY), and 6-deoxo-28-norcastasterone (6-deoxo-28-norCS) (Yokota et al., 2001; Kim et al., 2004b). In addition, the cell-free enzyme extract of tomato seedlings catalysed the conversion of cholesterol to cholestanol and 6-deoxo-28-norTE to 28-norCS via 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY, and 6-deoxo-28-norCS. These findings demonstrate that the synthesis of 28-norCS is mediated by late C-6 oxidation (Kim et al., 2004b). Furthermore, the cell-free enzyme extract mediated the C-24 methylation of 28-norCS to CS in the presence of NADPH and S-adenosyl-L-methionine (SAM). It was also determined that exogenously applied 28-norCS restores the abnormal growth of the tomato dwarf mutant which is defective in a cytochrome P450, CYP85A, involved in the C-6 oxidation of 6-deoxoCS and 6-deoxo-28-norCS to CS and 28-norCS, respectively. Therefore, 28-norCS is biologically important per se and is also important in the production of CS.
In Arabidopsis, C27-BRs including 28-norTY and 28-norCS have been identified, in addition to the C28-BRs (Fujioka et al., 2000). The conversion of cholestanol to 6-oxocholestanol, as a possible upstream step in C27-BRs biosynthesis, has also been demonstrated (Lee et al., 2010), although downstream steps for the generation of C27-BRs in A. thaliana have yet to be clearly elucidated.
Despite our previous efforts, the biosynthesis of C27-BRs via the early C-6 oxidation pathway remains to be clearly characterized. Furthermore, the linkage of the early and late C-6 oxidation pathways of C27-BRs, as well as the biosynthetic relationship between C27- and C28-BRs, is still not completely understood. In this study, these subjects were investigated using Arabidopsis enzyme extracts. The enzymes and genes involved in C27-BRs biosynthesis have also been addressed.
Materials and methods
Plant growth conditions
Cold-treated seeds of wild-type Arabidopsis (Col-0) were planted in soil and grown for 3 weeks in an environmental growth chamber at 22 °C, under a 16 h light (120 μmol photons m−2 s−1)/20 °C, 8 h dark cycle. When seeds were planted on 1× MS medium (Duchefa, Haarlem, Netherlands) containing 0.8% (w/v) agar and 1% (w/v) sucrose, the seeds were surface-sterilized with 70% ethanol and a 30% (v/v) bleach solution containing 0.025% (v/v) Triton X-100.
Enzyme assays
3-week-old soil-grown Arabidopsis plants (20 g) were harvested and ground with a mortar and pestle in cold 0.1 M sodium phosphate (pH 7.4) buffer containing 15 mM 2-mercaptoethanol, 1 mM EDTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulphonyl fluoride, 40 mM ascorbate, 250 mM sucrose, and 10% (v/v) glycerol. The homogenate was then centrifuged for 15 min at 8000 g to remove cell debris. The supernatant was then centrifuged for an additional 30 min at 20 000 g. The resultant supernatant was precipitated via the addition of cold acetone to a final concentration of 40% (v/v). The supernatant–acetone mixture was maintained for 10 min at –20 °C and centrifuged for an additional 10 min at 13 000 g. The resultant precipitate was dissolved in assay buffer containing 0.1 M sodium phosphate (pH 7.4) containing 1.5 mM 2-mercaptoethanol and 20% (v/v) glycerol, and used as the cell-free enzyme solution. For microsomal preparation, the supernatant obtained from centrifugation at 20 000 g was subjected to 1 h of ultra-centrifugation at 100 000 g. The resultant pellet was re-suspended with assay buffer.
The enzyme assay mixture was composed of 5 μg of substrate, 3–-5 mg of enzyme solution, and the appropriate co-factor (NADP/NADPH) or co-substrate (S-adenosyl-L-methionine). The reactions were initiated via the addition of substrate and the incubation was conducted for 30 min at 37 °C. The metabolites of the enzyme reactions were extracted with ethyl acetate (1.2 ml, three times) and concentrated in vacuo. The ethyl acetate-soluble fraction was loaded onto a Sep-Pak C18 cartridge column (Waters, Milford, MA), and sequentially washed with 50% and 60% methanol (5 ml each). The fraction eluted with 100% methanol was concentrated in vacuo, dissolved in 50 μl of methanol, and then subjected to reversed phase (RP)-HPLC (Senshu Pak C18, 10×150 mm) eluted at a flow rate of 2.5 ml min−1 with 100% methanol for the metabolites of cholesterol and cholestanol or acetonitrile (MeCN)–water gradients (0–20 min, 45% MeCN; 20–40 min, 45–100% MeCN; 40–70 min, 100% MeCN) for 6-deoxo-28-norBRs, or a flow rate of 2 ml min−1 with 60% MeCN for 28-norBRs. The fractions were collected every minute. The fractions (cholestanol, 18–19 min; 6-oxo-cholestanol, 7–8 min; 6-deoxo-28-norCT, 58–61 min; 6-deoxo-28-norTE, 44–46 min; 6-deoxo-28-nor-3-DHT, 46–48 min; 6-deoxo-28-norTY, 49–51 min; 6-deoxo-28-norCS, 36–38 min, 28-norTE, 27–29 min; 28-nor-3-DHT, 34–36 min; 28-norTY, 33–35 min; 28-norCS, 13–15 min) in which authentic BRs were detected under the same RP-HPLC conditions were analysed via GC-MS or GC-SIM after appropriate derivatization.
C-6 oxidations of C27-and C28-BRs by CYP85A1 and CYP85A2
CYP85A1/V60/WAT21 and CYP85A2/V60/WAT21 yeast strains were employed as previously described (Kim et al., 2005b). 6-Deoxo-28-norBR and its counterpart, 6-deoxo-BR, (5 μg each) were fed to galactose-induced yeast cells and incubated for 6 h. [26,28-2H6]BR was added to the cell culture as an internal standard prior to extraction with ethyl acetate. Purification using a Sep-Pak C18 cartridge column was conducted in accordance with the method described above. The fraction eluted with 100% methanol was subjected to RP-HPLC (Senshu Pak C18, 10×150 mm) eluted at a flow rate of 2.5 ml min−1 with MeCN–water gradients (0–20 min, 45% MeCN; 20–40 min, 45–100% MeCN; 40–60 min, 100% MeCN). The fractions (28-norTE, 27–29 min; 28-nor-3-DHT, 34–36 min; 28-norTY, 33–35 min; 28-norCS, 13–15 min; TE, 31–34 min; 3-DHT, 37–39 min; TY, 37–39 min; CS, 19–21 min) containing C27-BR and C28-BR were eluted and combined, and then subjected to GC-MS analysis. The quantities of the C28-BRs metabolites were initially calculated using [26,28-2H6]BRs as an internal standard and the amounts of C27-BRs, the counterparts of C28-BRs, were estimated by the area ratio relative to C28-BRs on the total ion chromatogram.
Sterol analysis
3-week-old soil-grown Arabidopsis plants (2 g fresh weight) were harvested and extracted with methanol:chloroform (4:1, v/v). The extracts were concentrated in vacuo and solvent-partitioned between chloroform and water. D7 cholesterol (0.5 μg) was added to the chloroform-soluble fraction as an internal standard. The fraction extracted with n-hexane after alkaline hydrolysis was purified on a Sep-Pak silica cartridge column (Waters, Milford, MA) and subjected to GC-MS analysis.
GC-MS/SIM analysis
The GC-MS or GC-SIM analyses were conducted as previously described (Kim et al., 2005b). The samples were subjected to methaneboronation or trimethylsilyation according to the structures of the expected metabolites. Methaneboronation was conducted by heating the samples dissolved in pyridine containing methaneboronic acid (2 mg ml−1) at 80 °C for 30 min and N-methyl-N-TMS-trifluoroacetamide (MSTFA, Pierce, Rockford, IL) was used for trimethylsilylation.
Rescue experiments of det2 and dwf4 mutants by C27-BRs
The det2, dwf4, and wild-type (Col-0 or En-2) seeds were surface-sterilized and planted on 1× MS agar plates containing 1 μM of various C27-BR biosynthetic intermediates or mock solution. After 5 d under continuous darkness, the seedlings (n >30) were photographed with a digital camera and the lengths of the hypocotyls were measured with Scion Image software (Scion Corporation, Maryland, USA).
Results
Arabidopsis enzymes were extracted with phosphate buffer containing the appropriate additives prior to centrifugation, and successive precipitation with acetone. The precipitates were then dissolved in assay buffers and employed as crude enzyme extracts for in vitro conversion experiments. Unlabelled substrates were used for enzymatic incubation, since isotope-labelled substrates were not available. The absence of the expected products in the prepared enzyme extracts was confirmed via GC-MS and GC-SIM prior to incubation with the substrates. The enzyme products were purified via RP-HPLC and then derivatized to trimethylsilyl ethers (TMSi), bismethaneboronates (BMB) or methaneboronate-trimethylsilyl ethers (MB-TMSi). These derivatives were rigorously characterized by GC-MS and/or GC-SIM analyses.
Biosynthesis 6-deoxo C27-BRs in A. thaliana
The late C-6 oxidation pathway for 28-norCS proceeds through the following sequence: cholesterol→cholestanol→6-deoxo-28-norCT→6-deoxo-28-norTE→6-deoxo-28-nor-3-DHT→6-deoxo-28-norTY→6-deoxo-28-norCS→28-norCS. The presence of this pathway has been demonstrated, although not fully clarified, in the tomato. By way of contrast, no evidence has yet been obtained supporting the existence of such a pathway in A. thaliana.
Our Arabidopsis enzyme extracts catalysed the conversion of cholesterol to cholestanol, which is consistent with our findings that cholesterol and cholestanol are endogenous in Arabidopsis plants (Table 1). However, the incubation of cholestanol and 6-deoxo-28-norCT in the crude Arabidopsis enzyme extract did not result in any of the expected metabolites. However, enzymes prepared from microsomes catalysed the conversion of cholestanol to 6-deoxo-28-norCT and 6-deoxo-28-norTE (Table 2). The enzymes that convert cholestanol to 6-deoxo-28-norCT and 6-deoxo-28-norTE do not appear to be abundant in A. thaliana. On the other hand, the crude enzyme extract catalysed the conversion of 6-deoxo-28-norTE to 6-deoxo-28-nor-3-DHT and 6-deoxo-28-norTY, of 6-deoxo-28-nor-3-DHT to 6-deoxo-28-norTE and 6-deoxo-28-norTY, and of 6-deoxo-28-norTY to 6-deoxo-28-nor-3-DHT and 6-deoxo-28-norTE; these results indicate that the epimerization of C-3 from 6-deoxo-28-norTE to 6-deoxo-28-norTY occurs via 6-deoxo-28-nor-3-DHT, in a reversible fashion. The metabolites of 6-deoxo-28-norTY also included 6-deoxo-28-norCS, demonstrating that the enzyme extract harbours C2 α-hydroxylase. Finally, 6-deoxo-28-norCS was metabolized to 28-norCS by the same enzyme extract. It has also been established that the late C-6 oxidation pathway, which produces 28-norCS, is operant in A. thaliana, as anticipated.
Table 1.
Content of major 4-demethylasterols in A. thaliana
| Amount (μg g−1 fresh weight) | ||
| 1st experiment | 2nd experiment | |
| Cholesterol | 6.60 | 7.94 |
| Cholestanol | 0.38 | 1.45 |
| Campesterol | 22.41 | 21.06 |
| Campestanol | 1.21 | 1.02 |
| Stigmasterol | 3.62 | 4.84 |
| Sitosterol | 107.13 | 99.45 |
| Sitostanol | 8.35 | 9.68 |
Table 2.
GC-MS data of metabolites obtained from A. thaliana cell-free conversion experiments
| Substrate | Metabolite | RRta | Prominent Ions |
| CHR | CHNb | 0.456 | 460(M+, 54), 445(73), 370(28), 355(43), 305(33), 215(100) |
| CHN | 6-oxoCHNc | 0.495 | 474 (M+, 19), 459(51), 445(100), 384(4), 159(8) |
| 6-deoxo-28-norCT | 0.542 | 533(M+-15, 1), 368(2), 255(8), 173(100) | |
| 6-deoxo-28-norTE | 0.605 | 516(M+, 69), 501(55), 459(23), 426(26), 411(39), 305(35), 230(27), 215(100), 141(30) | |
| 28-norTE | 28-nor-3-DHT | 0.876 | 456(M+, 90), 399(3), 316(19), 286(13), 245(35), 141(100) |
| 28-norTY | 0.735 | 530(M+, 60), 515(35), 501(100), 440(56), 425(21), 229(16), 141(21) | |
| TE | 1.031 | 544(M+, 29), 529(53), 515(100), 454(5), 300(8), 155(39) | |
| 28-nor-3-DHT | 3-DHT | 1.013 | 470(M+, 63), 399(7), 357(5), 316(21), 298(10), 287(11), 245(11), 155(100) |
| 28-norTY | 28-nor-3-DHT | 0.876 | 456(M+, 90), 399(2), 316(16), 286(11), 245(36), 141(100) |
| 28-norTE | 0.906 | 530(M+, 21), 515(53), 501(100), 440(3), 316(16), 141(11) | |
| 28-norCS | 0.866 | 498(M+, 100), 483(8), 399(4), 358(12), 328(7), 287(36), 141(52) | |
| TY | 0.863 | 544(M+, 100), 529(81), 515(55), 454(72), 300(10), 155(60) | |
| 28-norCS | CS | 1.000 | 512(M+, 80), 358(33), 327(12), 287(32), 155(100) |
| 6-deoxo-28-norTE | 6-deoxo-28-nor-3-DHT | 0.615 | 442(M+, 73), 427(10), 246(12), 231(100), 217(23), 163(20), 141(15) |
| 6-deoxo-28-norTY | 0.523 | 516(M+, 23), 501(6), 459(4), 426(62), 411(60), 305(11), 230(30), 215(100), 141(24) | |
| 28-norTE | 0.906 | 530(M+, 21), 515(50), 501(100), 440(5), 316(18), 141(12) | |
| 6-deoxo-28-nor-3-DHT | 6-deoxo-28-norTE | 0.605 | 516(M+, 73), 501(65), 459(25), 426(23), 411(36), 305(38), 230(26), 215(100), 141(17) |
| 6-deoxo-28-norTY | 0.523 | 516(M+, 21), 501(5), 459(4), 426(60), 411(59), 305(10), 230(32), 215(100), 141(24) | |
| 28-nor-3-DHT | 0.876 | 456(M+, 91), 399(3), 316(20), 286(13), 245(33), 141(100) | |
| 6-deoxo-28-norTY | 6-deoxo-28-nor-3-DHT | 0.615 | 442(M+, 74), 427(10), 246(12), 231(100), 217(22), 163(20), 141(14) |
| 6-deoxo-28-norTE | 0.605 | 516(M+, 71), 501(62), 459(25), 426(23), 411(35), 305(36), 230(26), 215(100), 141(23) | |
| 28-norTY | 0.735 | 530(M+, 60), 515(34), 501(100), 440(55), 425(24), 229(15), 141(23) | |
| 6-deoxo-28-norCS | 0.619 | 484(M+, 51), 469(16), 288(15), 273(100), 205(24), 141(21) | |
| 6-deoxo-28-norCS | 28-norCS | 0.866 | 498(M+, 100), 483(3), 399(4), 358(12), 328(7), 287(36), 141(54) |
RRt: relative retention time on GC.
The sample was analysed as BMB.
The sample was analysed as BMB-TMSi ether.
Biosynthesis of 6-oxo C27-BRs in A. thaliana
If the early C-6-oxidation pathway of C27-BRs exists in A. thaliana, 28-norCS will be synthesized according to the following sequence: cholestanol→6-oxocholestanol→28-norCT→28-norTE→28-nor-3-DHT→28-norTY→28-norCS. It was determined that our Arabidopsis enzyme extracts catalysed the conversion of cholestanol to 6-oxocholestanol (Table 2). However, 28-norCT was not detected in the cholestanol metabolites, and thus this metabolism was investigated further using enzymes prepared from microsomes obtained via ultra-centrifugation. Nonetheless, 28-norCT, as well as further metabolites including 28-norTE, were not produced in the reaction mixture as shown by the results of GC-SIM analysis. It appears most likely that the pathway from 6-oxocampestanol to 28-norCT is blocked in Arabidopsis.
The feeding of 28-norTE to the enzyme extract resulted in the production of 28-nor-3-DHT and 28-norTY, whereas the feeding of 28-norTY gave rise to 28-nor-3-DHT and 28-norTE, thereby indicating that 28-norTE and 28-norTY are interconvertible via 28-nor-3-DHT (Table 2). Furthermore, 28-norCS was identified as another metabolite of 28-norTY (Table 2). Therefore, the pathway connecting 28-norTE to 28-noCS was determined to be present in A. thaliana.
Biosynthetic connection of 6-deoxo and 6-oxo C27-BRs in A. thaliana
Two parallel pathways—the early and late C-6 oxidation pathways of C28-BRs—are biosynthetically connected by the C-6 oxidation of 6-deoxoTE, 6-deoxo-3-DHT, and 6-deoxoTY to TE, 3-DHT, and TY, respectively. In Arabidopsis, AtBR6ox1 (CYP85A1) and AtBR6ox2 (CYP85A2) mediate these C-6 oxidations (Kim et al., 2005b). An attempt was made to determine whether CYP85A1 and CYP85A2 are involved in any possible biosynthetic connection between the early and late C-6 oxidation pathways of C27-BRs. To this end, the cDNA of Arabidopsis CYP85A1 and CYP85A2 were cloned into a galactose-inducible expression vector, pYeDP60 (V60), and transformed into the WAT21 yeast strain, wherein the expression of Arabidopsis NADPH-Cyt P450 reductase is inducible by galactose (Pompon et al., 1996; Urban et al., 1997). After confirming that the C-6 oxidation of 6-deoxo-28-norCS did not occur in the empty vector-transformed yeast (V60/WAT21), 6-oxidations of C27- and C28-BRs were evaluated by the transformed strains (CYP85A1/V60/WAT21 and CYP85A2/V60/WAT21).
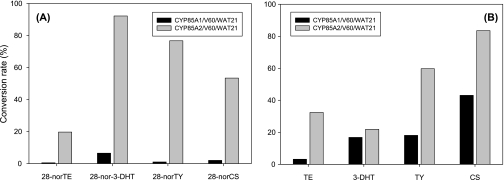
Both CYP85A1/V60/WAT21 and CYP85A2/V60/WAT21 successfully catalysed the 6-oxidation of C27-BRs, 6-deoxo-28-norTE, 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY, and 6-deoxo-28-norCS to 28-norTE, 28-nor-3-DHT, 28-norTY, and 28-norCS, respectively (Fig. 2A). The respective conversion rates were 17, 14, 68, and 24 times higher in CYP85A2/V60/WAT21 than in CYP85A1/V60/WAT21.
Fig. 2.
Comparison of BR C-6 oxidase activity in CYP85A1/V60/WAT21 and CYP85A2/V60/WAT21 strains. (A) C-6 oxidation for C27-BRs, (B) C-6 oxidation for C28-BRs.
Similarly, when C28-BRs, 6-deoxoTE, 6-deoxo-3-DHT, 6-deoxoTY, and 6-deoxoCS were fed to the transformed yeast strains, TE, 3-DHT, TY, and CS were detected as the respective products (Fig. 2B). The rates of conversion by CYP85A2/V60/WAT21 were also higher than the rates of conversion by CYP85A1/V60/WAT21, although to lesser extents than were noted in the 6-oxidations of C27-BRs. No 6-oxidations occurred in the feedings of the biosynthetically upstream intermediates, campestanol, cholestanol, 6-deoxoCT, and 6-deoxo-28-norCT.
An attempt was also made to determine whether Arabidopsis enzyme extracts are capable of converting 6-deoxo C27-BRs to 6-oxo C27-BRs. As shown in Table 2, 6-deoxo-28-norTE, 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY, and 6-deoxo-28-norCS were 6-oxidized to 28-norTE, 28-nor-3-DHT, 28-norTY, and 28-norCS, respectively. However, the 6-oxidation of 6-deoxo-28-norCT to 28-norCT was not detected in the enzyme extracts. These findings are consistent with those obtained using the transformed yeast strains.
Demethylation of 28-norCS
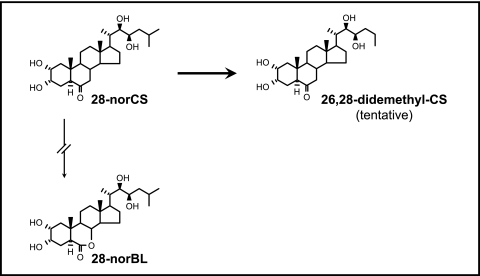
An attempt was made first to characterize the Arabidopsis enzymatic activity that converts 28-norCS to 28-norBL. However, we were unable to find any such an activity in the enzyme extract. Rather, it was determined that 28-norCS was converted to a compound with a molecular ion of m/z 484 as a BMB derivative. The molecular ion was 14 mass units smaller than that of the 28-norCS BMB derivative, which suggests the loss of a methyl group (Table 2). A prominent ion at m/z 127, which is derived from the side chain due to the fission of the C20–C22 bond, is also 14 mass units smaller than the corresponding ion of 28-norCS BMB. The presence of ions at m/z 358, 328, and 287 shows the ring structure to be identical to that of 28-norCS. It is, therefore, likely that one of the methyls was lost in the side chain. The loss of C-26 has been reported in previous metabolic studies of BRs (Kim et al., 2000, 2004a). Thus, the most probable structure of this metabolite is 26,28-dinorCS (Fig. 3).
Fig. 3.
Metabolism of 28-norCS in Arabidopsis. 28-NorCS converted to 26,28-didemethyl-CS (tentative), but not to 28-norBL.
Conversion of C27-BRs to C28-BRs through C-24 methylation
C27-BRs were incubated with the Arabidopsis enzyme extracts in the presence of S-adenosyl-L-methionine and NADPH, and their conversion to C28-BRs was assessed. The administration of 28-norCS yielded CS, as shown by a full-scan mass spectrum (Table 2). The administration of 28-norTE, 28-nor-3-DHT, and 28-norTY generated TE, 3-DHT, and TY, respectively, as identified by GC-SIM. Their conversion rates ranged from 0.2–0.3%, and were approximately 20–30-fold lower than that of the C-24 methylation of 28-norCS to CS (Table 3).
Table 3.
GC-MS/SIM data for C24-methylation of 28-norTE, 28-nor-3-DHT, 28-norTY, 28-norCS to TE, 3-DHT, TY, and CS in the presence of SAM and NADPH
| Substrate | Metabolite | Conversion rate (%) |
| 28-norTE | TE | 0.2 |
| 28-nor-3-DHT | 3-DHT | 0.2 |
| 28-norTY | TY | 0.3 |
| 28-norCS | CS | 6.0 |
Growth recovery by and biosyntheses of C28-BRs in the Arabidopsis mutants det2 and dwf4
The restoration of growth in the BR-deficient mutants, det2 and dwf4, was evaluated in dark-grown seedlings via the application of C27-sterols and C27-BRs.
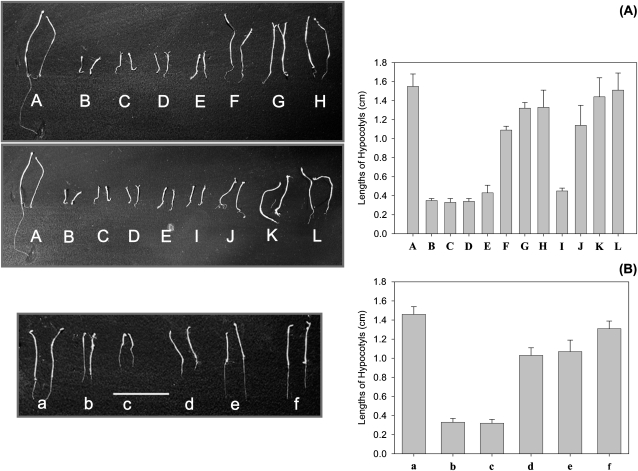
The det2 mutant was rescued by biosynthetically downstream C27-BRs in the early and late C-6 oxidation pathway, such as 6-deoxo-28-norCT, 6-deoxo-28-norTE, 6-deoxo-28-norTY, 28-norTE, 28-norTY, and 28-norCS, with more downstream BRs being more biologically active (Fig. 4A). A similar growth recovery rate was also observed in the dwf4 mutant (Fig. 4B).
Fig. 4.
Growth recovery of det2 (A) and dwf4 (B) by C27-sterols and C27-BRs. (A) A, Col-0/B-L, det2; B, Control; C, det2+cholesterol; D, det2+Cholest-4-en-3-one; E, det2+cholestanol; F, det2+6-deoxo-28-norCT; G, det2+6-deoxo-28-norTE; H, det2+6-deoxo-28-norTY; I, det2+6-oxocholestanol; J, det2+28-norTE; K, det2+28-norTY; L, det2+28-norCS. (B) a, Wild-type (En-2); b, dwf4; c, dwf4+6-oxocholestanol; d, dwf4+28-norTE; e, dwf4+28-norTY; f, dwf4+28-norCS. Error bars donate standard errors (n >30).
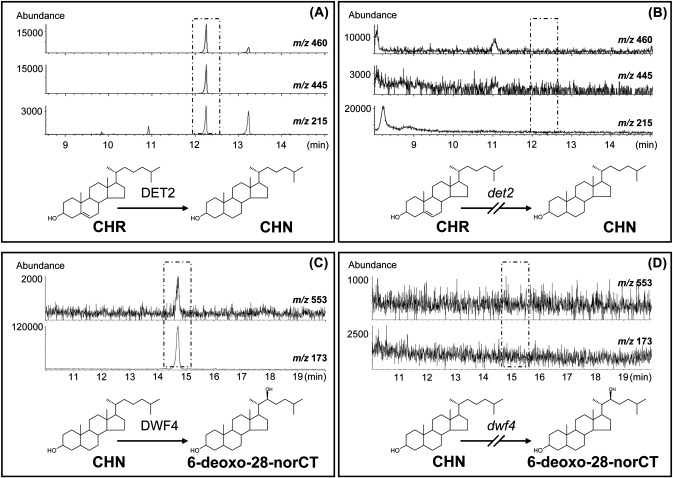
In an effort to investigate the role of the DET2 gene encoding for steroid 5α-reductase in C27-BRs biosynthesis, enzyme extracts were prepared from the wild-type Col-0 and the mutant det2, and were fed on cholesterol. The conversion of cholesterol to cholestanol was detected in Col-0 (Fig. 5A) but not in det2 (Fig. 5B), thereby indicating that the DET2 gene is involved in the conversion of cholesterol to cholestanol, and hence in the biosynthesis of C27-BRs.
Fig. 5.
GC-SIM analysis conversion of cholesterol to cholestanol and cholestanol to 6-deoxo-28-norCT in det2 (B) and dwf4 (D), respectively. (A) Conversion of cholesterol to cholestanol in Col-0, the wild type of det2. (C) Conversion of cholestanol to 6-deoxo-28-norCT in En-2, the wild type of dwf4.
The DWF4 gene encoding for steroid 22-hydroxylase was also evaluated for the conversion of cholestanol to 6-deoxo-28-norCT using the enzyme extract prepared from the wild-type En-2 and the mutant dwf4. The enzyme extract from En-2 successfully catalysed the conversion of cholestanol to 6-deoxo-28-norCT (Fig. 5C), but that from dwf4 did not (Fig. 5D), thereby indicating that the DWF4 gene is involved in the biosynthesis of C27-BRs.
Discussion
It was reported previously that the endogenous level of 28-norCS (0.24 ng g−1 fresh weight) in Arabidopsis reaches a level approximately one-eighth that of CS (2.01 ng g−1 fresh weight) (Kim et al., 2005b). Furthermore, it has been demonstrated that a change as small as 20% in the endogenous level of CS can induce phenotypic alternations, thereby suggesting that C27-BRs including 28-norCS must play an important role in the growth and development of Arabidopsis (Kim et al., 2005b; Kwon et al., 2005). In seedlings of Arabidopsis, cholesterol, the parent sterol of 28-norCS, is contained at one-third the levels of campesterol, the parent sterol of CS (Table 1); this indicates that Arabidopsis contains a sufficient reservoir of cholesterol for use in the synthesis of 28-norCS.
Biosynthetic pathway of C27-BRs via late C-6 oxidation
In this study, it has been demonstrated, using Arabidopsis seedlings, that the synthesis of 28-norCS from cholesterol occurs via the late C-6 oxidation pathway: cholesterol→cholestanol→6-deoxo-28-norCT→6-deoxo-28-norTE↔6-deoxo-28-nor-3-DHT↔6-deoxo-28-norTY→6-deoxo-28-norCS→28-norCS. The same biosynthetic pathway has been tentatively proposed in the tomato plant (Yokota et al., 2001).
Recent biochemical studies conducted by Ohnishi et al. (2006, 2009) have demonstrated that the CYP90B1-mediated 22-hydroxylation of campesterol is an important first step in the synthesis of C28-BRs in Arabidopsis. Campesterol and cholesterol were found to be favourable substrates for this enzyme, when compared with campestanol and cholestanol (Fujita et al., 2006). It is therefore assumed that the formation of 22-hydroxycholesterol from cholesterol is an important step in 28-norCS biosynthesis. The biosynthesis of C27-BRs, starting with the 22-hydroxylation of cholesterol, is currently being investigated.
The early C-6 oxidation pathway of C27-BRs is blocked and 6-oxoBRs is derived from respective 6-deoxoBRs
It was determined that the early C-6 oxidation pathway halted at the stage of 6-oxocholestanol because its presumed metabolite, 28-norCT, was not generated after incubation with a microsomal enzyme preparation. In addition, endogenous 28-norCT we could not be identified, even using as much as 30 kg fresh weight of Arabidopsis plants (data not shown). However, Arabidopsis contained enzymes that converted 28-norTE to 28-nor-3-DHT, 28-norTY, and 28-norCS successively, thereby indicating that the BRs belonging to the early C-6 oxidation pathway are supplied by respective 6-deoxoBRs. Among the enzymes responsible for C-6 oxidation of C27-BRs, CYP85A2 was determined to be 15 times as active as CYP85A1 in the C-6 oxidation of C27-BRs, thereby indicating that CYP85A2 performs a central function in the C-6 oxidation of C27-BRs (Fig. 2A). CYP85A2 has been determined to be more powerful than CYP85A1 in the C-6 oxidation of C28-BRs (Kim et al., 2005b). CYP85A2 also exhibits BL synthase activity (Kim et al., 2005b; Kwon et al., 2005; Nomura et al., 2005). However, CYP85A2 did not catalyse the 7-oxalactonation of 28-norCS to 28-norBL, thereby suggesting that CYP85A2 is specific for the conversion of CS to BL.
Disproof against the early C-6 oxidation pathway of C28-BRs
Some evidence has accumulated against the notion that the early C-6 oxidation pathway plays a role in C28-BR biosyntheses. The first step of this pathway is the 6-oxidation of campestanol to 6-oxocampestanol, which has previously been identified from Catharanthus crown gall cells (Fujioka and Sakurai, 1997). However, since that time, the occurrence of 6-oxocampestanol in other plants has yet to be confirmed. It has been determined that CYP85A1 and CYP85A2, which are known as BR 6-oxidases, did not catalyse this reaction, leaving the responsible enzyme to be determined (Shimada et al., 2001; Kim et al., 2005b; Kwon et al., 2005). Furthermore, the 22-hydroxylation of 6-oxocampestanol to CT has yet to be confirmed even in Catharanthus crown gall cells, although CT was endogenous in the cells (Fujioka et al., 1995). The conversion of 6-oxocampestanol to CT, as well as the presence of CT in any other plants, has yet to be demonstrated (Fujioka et al., 1995; Joo et al., 2002). Recently, Fujita et al. (2006) demonstrated that DWF4 (CYP90B1) 22-hydroxylated campestanol, but not 6-oxocampestanol. Altogether, our results indicate that the early C-6 oxidation pathway is commonly interrupted in plant tissues.
Biosyntheses of C27- and C28-BRs are catalysed by the same enzymes
Biosynthetic reactions occurring in C27-BRs biosynthesis, including 5α-reduction, C-22 hydroxylation, C-23 hydroxylation, C-3 epimerization, C-2 α-hydroxylation, and C-6 oxidation, are exactly the same as those occurring in C28-BRs biosynthesis. This may suggest that the same enzymes mediate the same reactions in the biosyntheses of both C28-BRs and C27-BRs. In support of this notion, heterologously-expressed CYP85A1 and CYP85A2 involved in the C-6 oxidation of C28-BRs exert the same activity in the biosynthesis of C27-BRs. The det2 mutant cannot 5α-hydrogenate campesterol, and also cannot 5α-hydrogenate cholesterol (Fig. 5B), whereas the dwf4 mutant catalyses the 22R-hydroxylation of neither campestanol nor cholestanol. Moreover, the abnormal growth of det2 and dwf4 mutants was successfully restored via the exogenous application of downstream C27-BRs. Collectively, the findings of this study suggest that C27-BRs and C28-BRs biosynthesis are most likely controlled by the same biosynthetic enzymes.
CS synthesis from 28-norCS via methylation
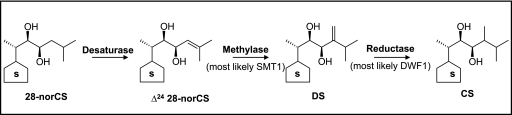
It has been determined that Arabidopsis enzyme extract can methylate 28-norCS to CS. This constitutes a supplement to our earlier report demonstrating the presence of the same enzymatic activity in the tomato (Kim et al., 2004b). It appears that this methylation reaction may be a ubiquitous event in the plant kingdom. As shown in Fig. 6, using the tomato plant, it was determined that this reaction occurs via the following three steps: (i) desaturation of 28-norCS to form Δ24-28-norCS, (ii) SAM-dependent methylation of Δ24-28-norCS to form dolichosterone (DS), and (iii) NADPH-dependent reduction of DS by NADPH to form CS (Kim et al., 2004b). The SAM-dependent methylation is presumed to be catalysed by sterol methyltransferase 1 (SMT1). The NADPH-dependent reduction will be controlled by the DWF1 gene in Arabidopsis or its orthologue gene, OsDWF2, in rice. In support of this, the Osdwf2 rice mutant accumulates DS (Hong et al., 2005). Additional evidence was recently obtained, using a P. vulgaris enzyme extract, that NADPH is required for the conversion of DS to CS (Joo et al., 2009). It was found that 28-norCS is far more readily methylated than 28-norTE and 28-norTY in Arabidopsis, which indicates that C27-BRs and C28-BRs are connected largely through the passage from 28-norCS to CS (Fig. 1). In order to confirm the presence of these steps in Arabidopsis, metabolic and molecular genetic studies using relevant mutants are currently underway.
Fig. 6.
A proposed scheme for the three step C-24 methylation of 28-norCS to CS in the presence of SAM and NADPH in Arabidopsis. S indicates the same ring structure as that of 28-norCS and CS.
Deactivation of 28-norCS through demethylation
28-NorCS fed to the Arabidopsis enzyme extract was not only methylated, but also demethylated. It has been demonstrated previously in several plants that CS and BL are deactivated via C-26 demethylation into 26-norCS and 26-norBL (Kim et al., 2000; 2004a). Therefore, the demethylation product of 28-norCS is tentatively designated as 26,28-norCS. Such demethylation events appear to perform a crucial role in regulating the levels of 28-norCS, which is regarded as biologically active per se (Kim et al., 2005a).
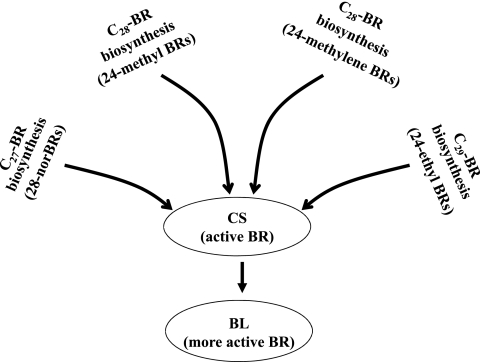
In conclusion, it has been demonstrated here that multiple biosynthetic pathways lead to CS. Recently, Joo et al. (2009) determined that, in P. vulgaris, DS is hydrogenated to CS. Our recent study (unpublished) revealed another biosynthetic pathway from 28-homoCS to CS via C-28 demethylation. Thus, it is most conceivable that all the biosynthetic pathways of BRs in plants are funnelled into CS to carry out the relevant biological activities (Fig. 7).
Fig. 7.
Biosynthetic connection of C27-BRs (28-norBRs), C28-BRs (24-methylene BRs and 24-methyl BRs) and C29-BRs (24-ethyl BRs) in plants. The multiple BRs biosynthetic pathways are funneled into CS to show BR activity in plant growth and development.
Acknowledgments
The authors would like to thank Dr S Takatsuto at the Joetsu University of Education for providing the authentic BRs and Drs D Pompon and P Urban for providing the pYeDP60 and the WAT21 yeast strain. This work was supported by a grant from the Next-Generation BioGreen21 Program (No. PJ007967) Rural Development Administration to S-KK and the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2009-353-C00021) to S-HJ in the Chung-Ang University, Republic of Korea.
References
- Bajguz A, Tretyn A. The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry. 2003;62:1027–1046. doi: 10.1016/s0031-9422(02)00656-8. [DOI] [PubMed] [Google Scholar]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JD, Kamiya Y. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proceedings of the National Academy of Sciences, USA. 1999;96:1761–1766. doi: 10.1073/pnas.96.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Yokota T. Plants steroid hormones, brassinosteroids: current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant and Cell Physiology. 2001;42:114–120. doi: 10.1093/pcp/pce018. [DOI] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22alpha-hydroxylation steps in brassinosteroid biosynthesis. The Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S. Natural occurrence of brassinosteroids in the plant kingdom. In: Sakurai A, Yokota T, Clouse SD, editors. Brassinosteroids: steroidal plant hormones. Tokyo: Springer-Verlag; 1999. pp. 21–45. [Google Scholar]
- Fujioka S, Inoue T, Takatsuto S, Yanagisawa T, Yokota T, Sakurai A. Identification of a new brassinosteroid, cathasterone, in cultured cells of Catharanthus roseus as a biosynthetic precursor of teasterone. Bioscience, Biotechnology and Biochemistry. 1995;59:1543–1547. [Google Scholar]
- Fujioka S, Li J, Choi YH, Seto H, et al. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. The Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Noguchi T, Sekimoto M, Takatsuto S, Yoshida S. 28-Norcastasterone is biosynthesized from castasterone. Phytochemistry. 2000;55:97–101. doi: 10.1016/s0031-9422(00)00261-2. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Sakurai A. Brassinosteroids. Natural Product Report. 1997;14:1–10. doi: 10.1039/np9971400001. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Yokota T. Biosynthesis and metabolism of brassinosteroids. Annual Review of Plant Biology. 2003;54:137–164. doi: 10.1146/annurev.arplant.54.031902.134921. [DOI] [PubMed] [Google Scholar]
- Fujita S, Ohnishi T, Watanabe B, Yokota T, Takatsuto S, Fujioka S, Yoshida S, Sakata K, Mizutani M. Arabidopsis CYP90B1 catalyses the early C-22 hydroxylation of C27, C28 and C29 sterols. The Plant Journal. 2006;45:765–774. doi: 10.1111/j.1365-313X.2005.02639.x. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. The Plant Cell. 2005;17:2243–2254. doi: 10.1105/tpc.105.030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S-H, Yun HS, Kim T-W, Kim Y-S, Kim S- K. Identification and transformation of campestanol in cultured cells of. Phaseolus vulgaris. Bulletin of the Korean Chemical Society. 2002;23:1035–1038. [Google Scholar]
- Joo S-H, Hwang J-Y, Park CH, Lee SC, Kim S- K. Biosynthetic connection of 24-methylene- and 24-methyl-brassinosteroids in. Phaseolus vulgaris. Bulletin of the Korean Chemical Society. 2009;30:502–504. [Google Scholar]
- Kim T-W, Chang SC, Choo J, Watanabe T, Takatsuto S, Yokota T, Lee JS, Kim S-Y, Kim S- K. Brassinolide and [26,28-2H6] brassinolide are differently demethylated by loss of C-26 and C-28, respectively, in Marchantia polymorpha. Plant, Cell and Physiology. 2000;41:1171–1174. doi: 10.1093/pcp/pcd048. [DOI] [PubMed] [Google Scholar]
- Kim T-W, Chang SC, Lee JS, Takatsuto S, Yokota T, Kim S- K. Novel biosynthetic pathway of castasterone from cholesterol in tomato. Plant Physiology. 2004b;135:1231–1242. doi: 10.1104/pp.104.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-W, Hwang J-Y, Joo S-H, Cheong HS, Pharis RP, Kim S- K. Endogenous level of 28-norcastasterone is strictly regulated in plant cells. Journal of Plant Biology. 2005a;48:483–486. [Google Scholar]
- Kim T-W, Hwang J-Y, Kim Y-S, Joo S-H, Chang SC, Lee JS, Takatsuto S, Kim S- K. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer–Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. The Plant Cell. 2005b;17:2397–2412. doi: 10.1105/tpc.105.033738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-W, Park H-H, Kim S- K. Cell-free conversion of castasterone in cultured cells of Phaseolus vulgaris and. Marchantia polymorpha. Bulletin of the Korean Chemical Society. 2004a;25:955–956. [Google Scholar]
- Koka CV, Cerny RE, Gardner RG, Noguchi T, Fujioka S, Takatsuto S, Yoshida S, Clouse SD. A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiology. 2000;122:85–98. doi: 10.1104/pp.122.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M, Fujioka S, Jeon JH, Kim HB, Takatsuto S, Yoshida S, An CS, Choe SH. A double mutant for the CYP85A1 and CYP85A2 genes of Arabidopsis exhibits a brassinosteroid dwarf phenotype. Journal of Plant Biology. 2005;48:237–244. [Google Scholar]
- Lee SC, Kim T-W, Hwang J-Y, Park CH, Son S-H, Youn JH, Kim S- K. Identification and biosynthesis of cholest-4-en-3-one and 6-oxocholestanol in young tomato plants. Bulletin of the Korean Chemical Society. 2010;31:1782–1784. [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH. Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science. 2002;295:1299–1301. doi: 10.1126/science.1065769. [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH, Vafeados D, Chory J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiology. 2001;127:14–22. doi: 10.1104/pp.127.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Takatsuto S, Sakurai A, Yoshida S, Li J, Chory J. Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-En-3-one to (24R)-24-methyl-5alpha-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiology. 1999;120:833–840. doi: 10.1104/pp.120.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Kitasaka Y, Takatsuto S, Reid JB, Fukami M, Yokota T. Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiology. 1999;119:1517–1526. doi: 10.1104/pp.119.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Kushiro T, Yokota T, Kamiya Y, Bishop GJ, Yamaguchi S. The last reaction producing brassinolide is catalyzed by cytochrome P-450s, CYP85A3 in tomato and CYP85A2 in Arabidopsis. Journal of Biological Chemistry. 2005;280:17873–17879. doi: 10.1074/jbc.M414592200. [DOI] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiology. 1997;113:31–37. doi: 10.1104/pp.113.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Szatmari AM, Watanabe B, et al. C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. The Plant Cell. 2006;18:3275–3288. doi: 10.1105/tpc.106.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Yokota T, Mizutani M. Insights into the function and evolution of P450s in plant steroid metabolism. Phytochemistry. 2009;70:1918–1929. doi: 10.1016/j.phytochem.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Pompon D, Louerat B, Bronine A, Urban P. Yeast expression of animal and plant P450s in optimized redox environments. Methods in Enzymology. 1996;272:51–64. doi: 10.1016/s0076-6879(96)72008-6. [DOI] [PubMed] [Google Scholar]
- Sakurai A. Biosynthesis. In: Sakurai A, Yokota T, Clouse SD, editors. Brassinosteroids: steroidal plant hormones. Tokyo: Springer-Verlag; 1999. pp. 137–136. [Google Scholar]
- Shimada Y, Fujioka S, Miyauchi N, Kushiro M, Takatsuto S, Nomura T, Yokota T, Kamiya Y, Bishop GJ, Yoshida S. Brassinosteroid 6-oxidases from Arabidopsis and tomato catalyze multiple C-6 oxidations in brassinosteroid biosynthesis. Plant Physiology. 2001;126:770–779. doi: 10.1104/pp.126.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Urban P, Mignotte C, Kazmaier M, Delorme F, Pompon D. Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. Journal of Biological Chemistry. 1997;272:19176–19186. doi: 10.1074/jbc.272.31.19176. [DOI] [PubMed] [Google Scholar]
- Yokota T. The structure, biosynthesis and function of brassinosteroids. Trends in Plant Science. 1997;2:137–143. [Google Scholar]
- Yokota T, Sato T, Takeuchi Y, Nomura T, Uno K, Watanabe T, Takatsuto S. Roots and shoots of tomato produce 6-deoxo-28-norcathasterone, 6-deoxo-28-nortyphasterol and 6-deoxo-28-norcastasterone, possible precursors of 28-norcastasterone. Phytochemistry. 2001;58:233–238. doi: 10.1016/s0031-9422(01)00237-0. [DOI] [PubMed] [Google Scholar]