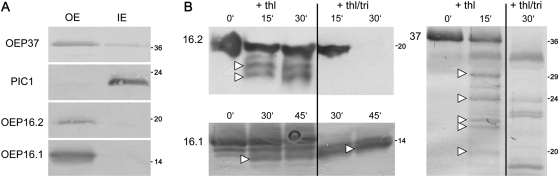
Fig. 2.
Localization of OEP16.1 and OEP16.2 in the plastid outer envelope membrane. For immunodetection of OEP16.2, envelope membranes were prepared from isolated chloroplasts of 7-day-old pea seedlings (all others: 10-day-old plants). Numbers indicate the molecular mass of proteins in kDa. (A) Immunoblot analysis of Ps-OEP16.1 and Ps-OEP16.2 in purified outer (OE) and inner (IE) envelope membranes from pea chloroplasts. Antisera against the proteins Ps-OEP37 (OE) and PIC1 (IE) were used as controls. Equal amounts of proteins were loaded per lane: for detection of OEP37, 5 μg; for PIC1, 20 μg; for OEP16.2, 30 μg; and for OEP16.1, 1 μg. (B) Outer envelope membrane vesicles from pea chloroplasts were protease digested with thermolysin (thl) and subsequently subjected to immunblot analysis for Ps-OEP16.2, Ps-OEP16.1, and Ps-OEP37. Thermolysin was incubated for 0–45 min in a 2:1 ratio (w/w) per μg of envelope proteins. To solubilize membranes, 1% Triton (+thl/tri) was added in control assays. For detection of OEP16.2 (upper panel) 90 μg, and for OEP16.1 (lower panel) and OEP37 (right panel) 2.5 μg of protein were loaded equally in each lane. Arrowheads indicate major digestion fragments. For the control OEP37, fragment sizes correspond to those detected by Schleiff et al. (2003).