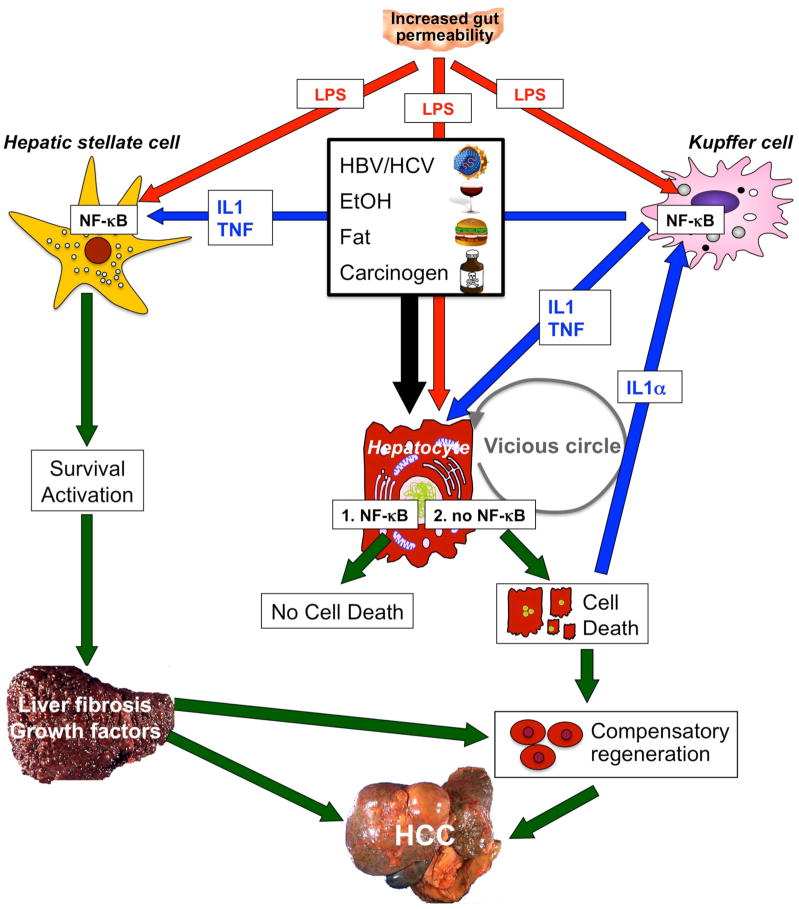
Figure 3.
NF-κB contributes to hepatocarcinogenesis in the setting of chronic injury, inflammation and fibrosis. Low levels of NF-κB exacerbate injury induced by hepatitis viruses, alcohol, fat, LPS or carcinogens. However, complete absence of NF-κB (achieved by deletion of Tak1 or Nemo in mouse models) is sufficient to initiate apoptosis of hepatocytes even without these triggers, probably because low levels of LPS still reach the liver. Increased hepatic injury leads to stimulation of regenerative responses in progenitor cells and activation of Kupffer cells by IL-1α released from dying hepatocytes. In turn, these processes stimulate NF-κB activation in Kupffer cells and the release of mediators such as IL-1β and TNF. These mediators may induce further hepatocyte injury, IL-1α release and regenerative responses, leading to a vicious circle of injury, inflammation and regeneration. At the same time, LPS from the intestinal microbiota and TNF and IL-1β from Kupffer cells act on HSCs to promote their activation and survival. Activated HSCs and/or hepatic myofibroblasts produce extracellular matrix, which changes the hepatic microenvironment. Abbreviations: HCC, hepatocellular carcinoma; HSC, hepatic stellate cell; IL, interleukin; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; TNF, tumor necrosis factor.