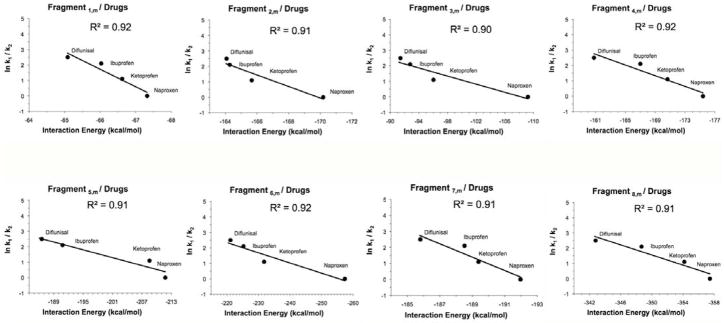
Figure 3.
The correlation of the experimental constants of competitive dissociation rate ln(ki/kj) (naproxen = 0, ketoprofen = 1.1, ibuprofen = 2.1, and diflunisal = 2.5), derived from CKM studies, between a PAMAM dendrimer and four NSAIDs, versus the average of the total interaction energies calculated using the methodology implemented in this work. The r2 value in all the graphs obtained was higher than 0.9, and the trend lines showed a correlation between the affinity degree of each drug by PAMAM and the theoretical values of the interaction energies calculated. Results showed the same relative affinity order observed experimentally for PAMAM–NSAIDs: naproxen > ketoprofen > ibuprofen > diflunisal.