Fig. 8.
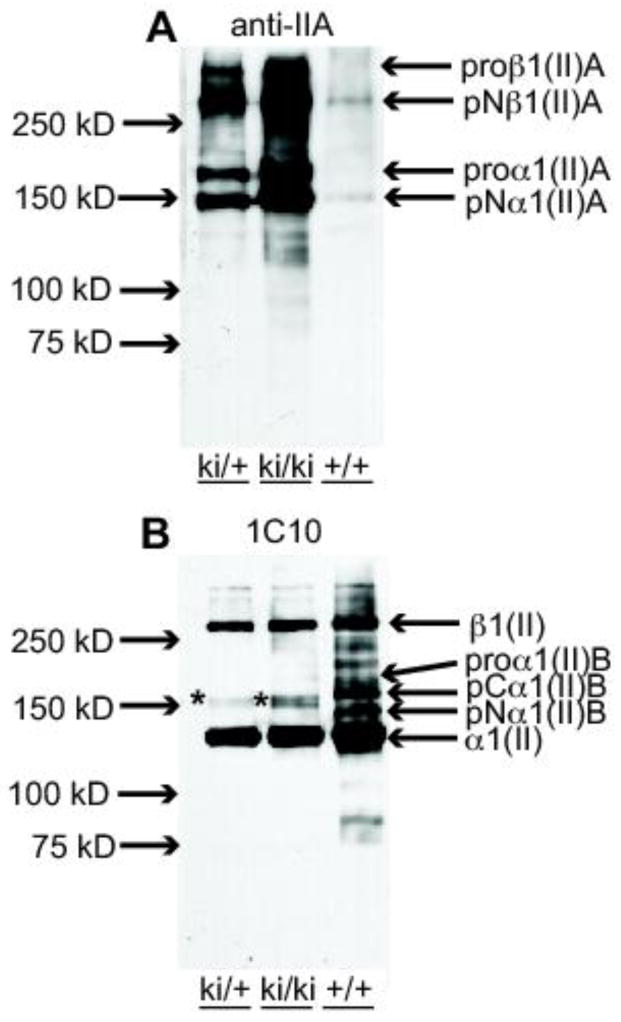
Analysis of type II procollagen processing in P7 epiphyseal cartilage. A. Western blot of 4M Gn-HCl extracts of epiphyseal cartilage from hindlimbs of P7 wild type (+/+), heterozygous (ki/+) and homozygous (ki/ki) mice probed with the anti-IIA polyclonal Ab (Oganesian et al., 1997). Weakly immunoreactive IIA-positive collagen bands in the 150 kDa to 250 kDa range are found in the +/+ lane; increasing intensities of these bands are found in the ki/+ and ki/ki bands, as expected. pNα1(II)A at 150 kDa represents procollagen molecules containing only the NH2-propeptide. The slower migrating band, proα1(II)A, represents procollagen chains containing both NH2- and COOH-propeptides. The two high molecular weight bands are dimers of the α1(II)A chains and indicated as proβ1(II)A and pNβ1(II)A as per nomenclature for collagen chains. B. Western blot of the same protein extracts described in A, but probed with monoclonal antibody 1C10 that recognizes native type II collagen (Fernandes et al., 2003). The major immunoreactive band in all three lanes is the fully processed α1(II) chain (devoid of NH2- and COOH-propeptides). The fully processed collagen chains in the ki/ki lane are derived only from type IIA procollagen. This antibody also reacts weakly with the 150 kDa band corresponding to pNα1(II)A (*). In the +/+ lane, type IIB procollagen processing intermediates are also detected, pCα1(II)B and pNα1(II)B. The highest molecular weight band, β1(II) represent dimers of the α1(II) chains. In ki/ki tissue, these dimers can only be derived from proα1(II)A collagen chains.
