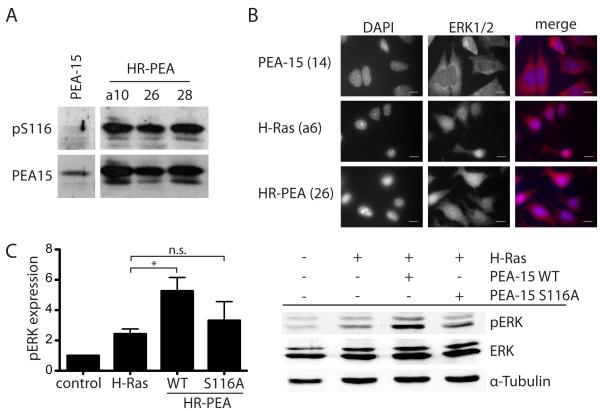
Figure 4.
PEA-15 is phosphorylated at Ser116 and does not sequester ERK in the cytoplasm when co-expressed with constitutively active H-Ras. (A) Western blot analysis of PEA-15 phosphorylation at Ser116 and total PEA-15 expression in stably transfected iBMK cells. (B) Immunofluorescence staining of ERK1/2 in stably transfected iBMK cell lines plated on fibronectin showing the cellular localization of ERK1/2 in these cells. Nuclei are visualized by DAPI staining. Both signals are also shown in a merged image. White scale bars represent 20μm.
Ser116 phosphorylation of PEA-15 is necessary for its enhancing effect on H-Ras induced activation of ERK. (C) Western blot analysis of pERK and total ERK in iBMK cells transiently expressing combinations of H-Ras, PEA-15 wildtype (WT) or a S116A mutant of PEA-15 that cannot be phosphorylated at Serine116. Cells were kept in suspension for 18h. Phosphorylation of ERK was normalized to total ERK expression. Shown is the mean and SEM of three independent experiments.