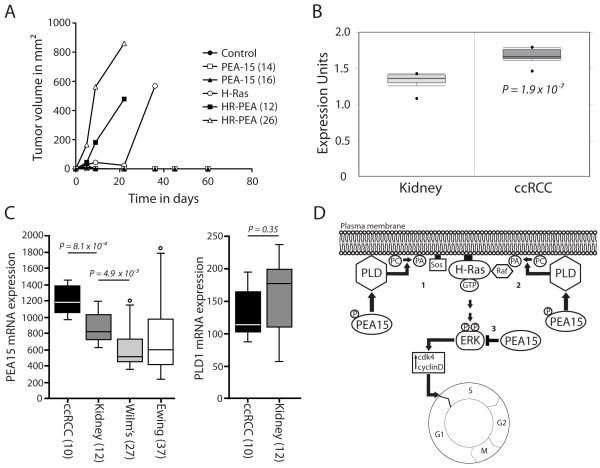
Figure 8.
PEA-15 co expression promotes earlier H-Ras tumor formation in nude mice in vivo; PEA-15 expression is elevated in human ccRCC samples. (A) Tumor formation in nude mice was followed after injection of iBMK cells stably expressing H-Ras, PEA-15, H-Ras/PEA-15 (HR-PEA) or control vector. Graphs represent changes in tumor volume from up to 60 days. Mice were sacrificed once the tumor burden was too great. (B) Differential expression analysis of PEA-15 levels in human kidney samples and human clear cell renal cell carcinoma samples (ccRCC) showing the relative protein expression units. Data was extracted from the Oncomine cancer microarray database. As a corrected measure of significance t-statistics with false discovery rates were used. (C) PEA15 and PLD1 expression in different tumor types and normal kidney. The data shown are from the Francesconi-37 Ewing sarcoma series (for Ewing), or from the Kort-79 mixed kidney series (for all other samples). Statistical analysis was performed using the non-parametric Kruskal-Wallis test, P < 0.05 was considered significant. The error bars represent the SEM. (D) Model of a proposed mechanism by which PEA-15 can increase H-Ras driven activation of ERK. Overexpression of PEA-15 in iBMK cells overexpressing constitutively active H-Ras causes higher PLD1 activity either through higher PLD1 expression levels or a yet undefined activation mechanism. Subsequently, PLD1 produces increased amounts of the signaling molecule phosphatidic acid (PA). PA then increases recruitment of the guanine nucleotide exchange factor SOS (1) and/or the kinase Raf (2) to the plasma membrane. H-Ras is constitutively bound to the plasma membrane and is immediately able to induce higher signaling rates upon GTP loading. This leads to the observed downstream effects like increased ERK activity and cell cycle progression. In this scenario PEA-15 is phosphorylated at Ser116. Unphosphorylated PEA-15 (3) is able to bind ERK and sequester it in the cytoplasm as previously reported.