Figure 2.
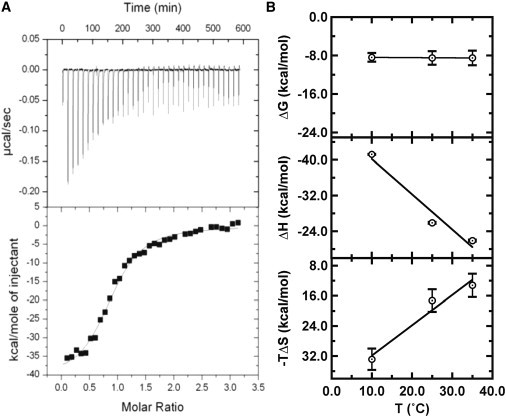
Representative ITC data. (A) (Upper) Thermograms showing formation of the HP2::HP3 kissing interaction. HP3 solution (60 μM) was titrated into 1.4 mL HP2 (3.5 μM) at 10°C in sodium-binding buffer. (Lower) Integrated data (solid squares) were fit to a single-site binding model (solid line), yielding: ΔH = −41.2 kcal mol−1; −TΔS = 32.8 kcal mol−1; KA = 2.8 × 106 M−1; n =1.0. (B) Thermodynamic energies ΔG (upper), ΔH (middle), and −TΔS (lower) as a function of temperature.
