Abstract
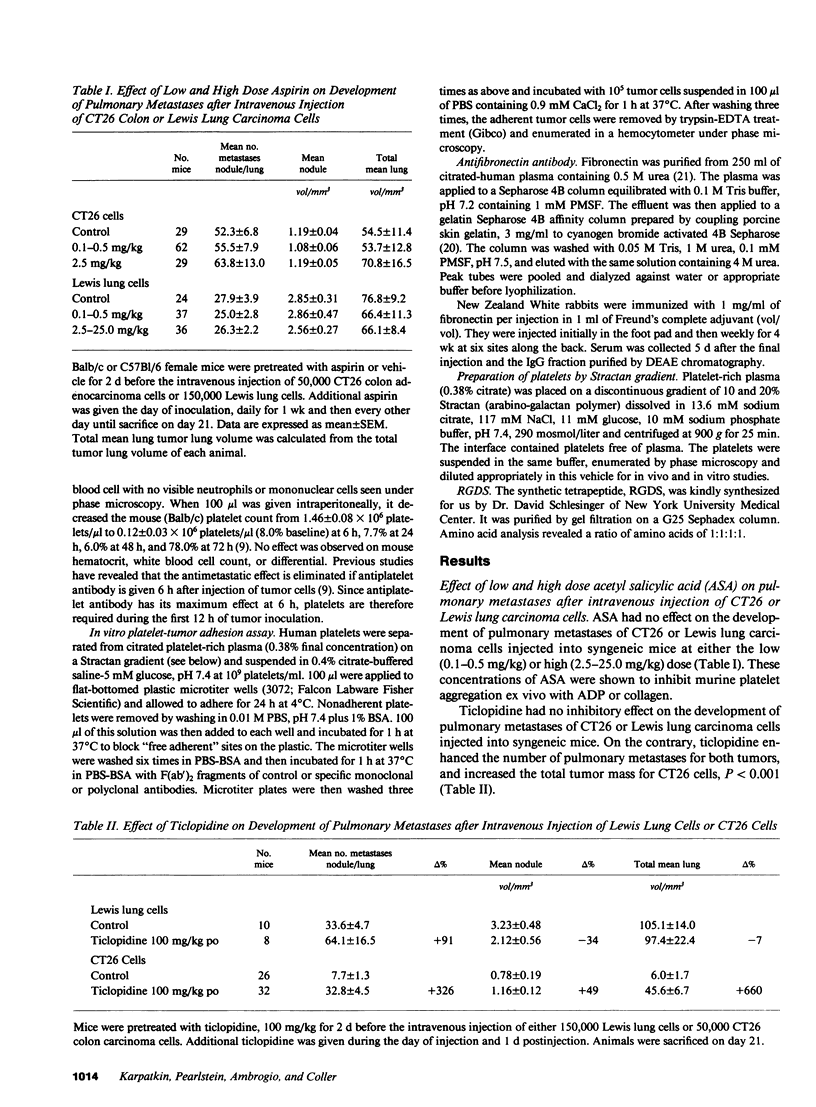
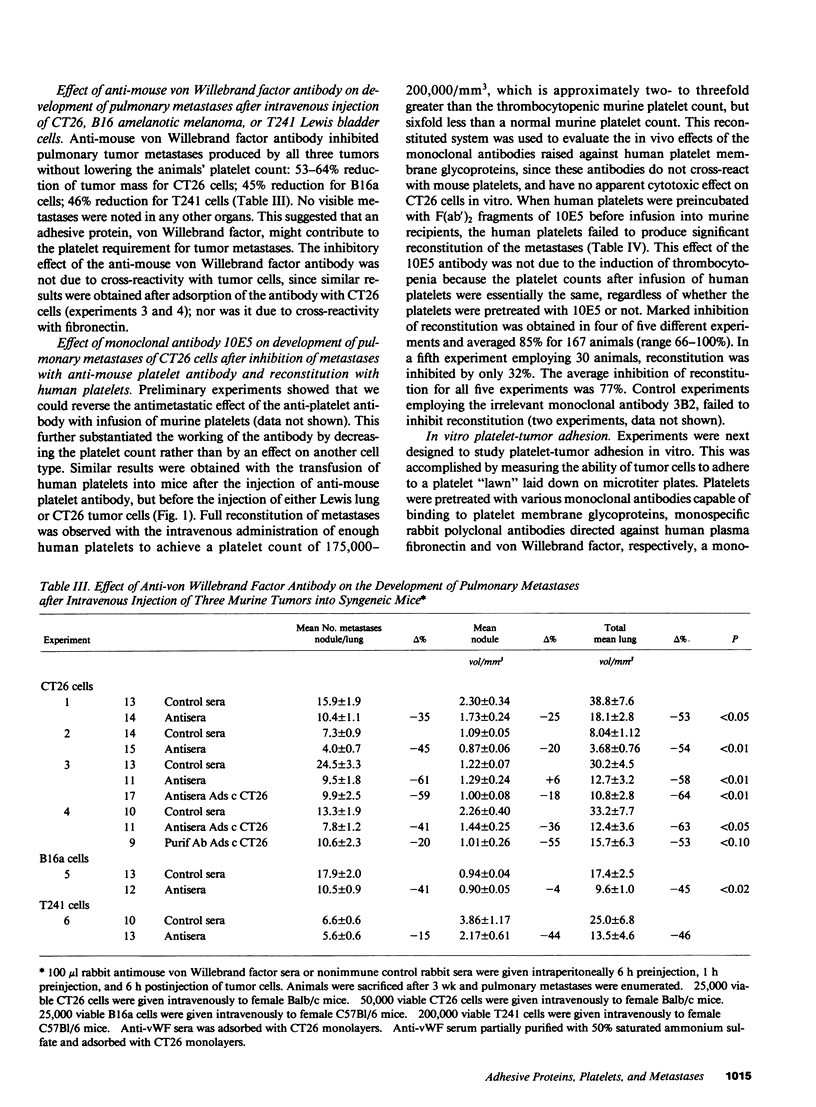
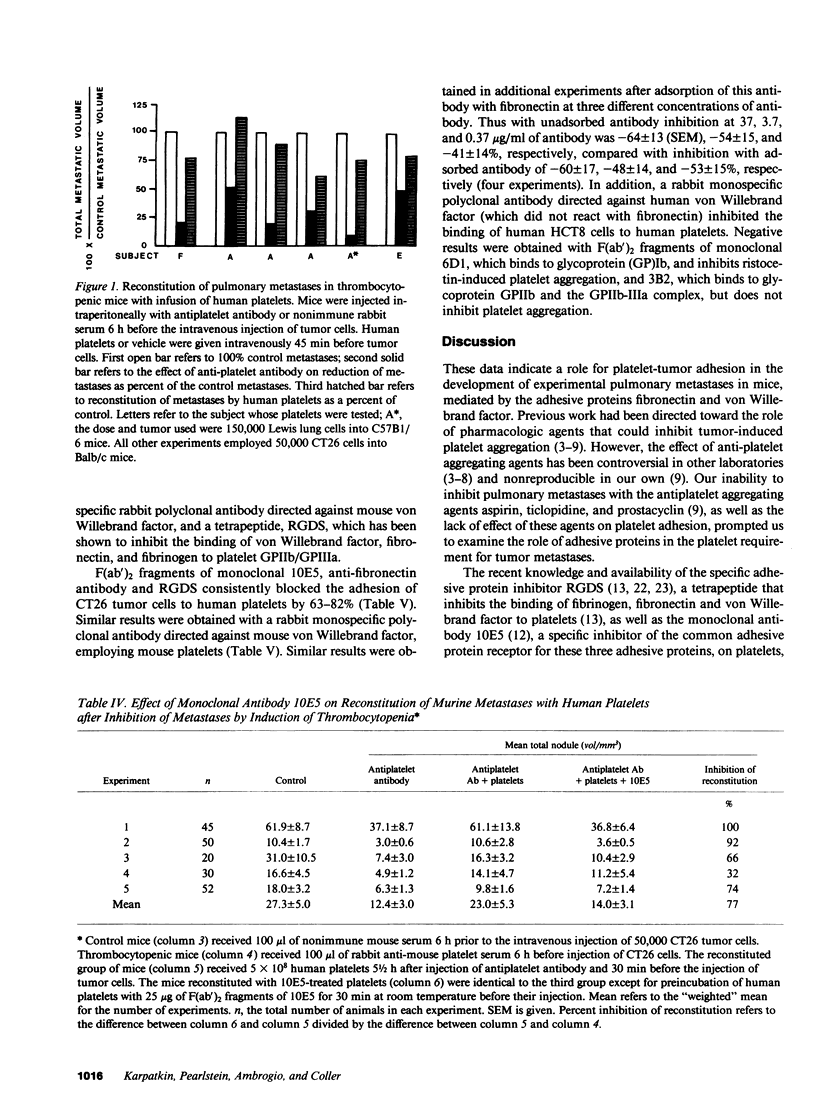
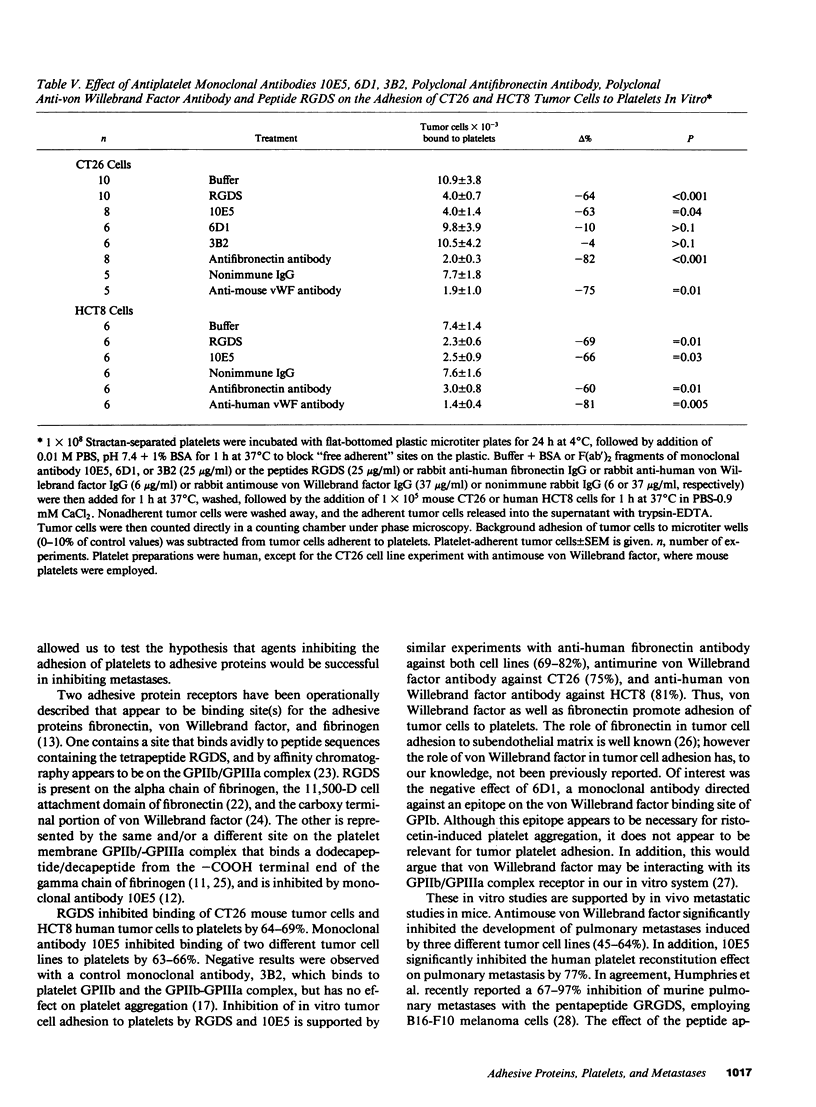
Platelet-adhesive protein-tumor cell interaction was studied in vitro and in vivo. Monoclonal antibody 10E5, which inhibits binding of fibronectin and von Willebrand factor to the platelet membrane glycoprotein GPIIb-GPIIIa complex, inhibited the binding of mouse CT26 and human HCT8 colon carcinoma cells to platelets by 63-65%, whereas an irrelevant monoclonal antibody, 3B2, had no effect. Monoclonal antibody 6D1, which inhibits binding of von Willebrand factor to GPIb, also had no effect. RGDS, a tetrapeptide that represents the adhesive domain of fibronectin and von Willebrand factor inhibited binding of the tumors to platelets by 64-69%. Monospecific polyclonal antifibronectin antibody inhibited binding by 60-82%; anti-von Willebrand factor antibody inhibited binding by 75-81%. In vivo, polyclonal monospecific anti-mouse von Willebrand factor antibody inhibited pulmonary metastases induced by CT26 tumor cells by 53-64%, B16a amelanotic melanoma cells by 45% and T241 Lewis bladder cells by 46% without induction of thrombocytopenia. Pulmonary metastases with CT26 cells could be inhibited by induction of thrombocytopenia, and reconstituted by infusion of either murine or human platelets. Reconstitution of pulmonary metastases with human platelets could be inhibited 77% by preincubation of human platelets with monoclonal antibody 10E5 before infusion of platelets into mice. Thus, platelets appear to contribute to metastases by their adhesive interaction with tumor cells via the adhesive proteins fibronectin and von Willebrand factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Galanti N., Johnson T., Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973 May;11(3):704–718. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Stewart C. C. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A. 1968 Sep;61(1):46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Witul M., Cohen H., Sciandra J., Williams P., Gastpar H., Murphy G. P., Ambrus J. L. Studies on platelet aggregation inhibitors in vivo. VIII. Effect of pentoxifylline on spontaneous tumor metastasis. J Med. 1979;10(6):435–443. [PubMed] [Google Scholar]
- Haverstick D. M., Cowan J. F., Yamada K. M., Santoro S. A. Inhibition of platelet adhesion to fibronectin, fibrinogen, and von Willebrand factor substrates by a synthetic tetrapeptide derived from the cell-binding domain of fibronectin. Blood. 1985 Oct;66(4):946–952. [PubMed] [Google Scholar]
- Hilgard P., Heller H., Schmidt C. G. The influence of platelet aggregation inhibitors on metastasis formation in mice (3LL). Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1976 Aug 30;86(3):243–250. doi: 10.1007/BF00286943. [DOI] [PubMed] [Google Scholar]
- Honn K. V., Cicone B., Skoff A. Prostacyclin: a potent antimetastatic agent. Science. 1981 Jun 12;212(4500):1270–1272. doi: 10.1126/science.7015512. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Karpatkin S., Ambrogio C., Pearlstein E. Lack of effect of in vivo prostacyclin on the development of pulmonary metastases in mice following intravenous injection of CT26 colon carcinoma, Lewis lung carcinoma, or B16 amelanotic melanoma cells. Cancer Res. 1984 Sep;44(9):3880–3883. [PubMed] [Google Scholar]
- Kelton J. G., Hirsh J., Carter C. J., Buchanan M. R. Thrombogenic effect of high-dose aspirin in rabbits. Relationship to inhibition of vessel wall synthesis of prostaglandin I2-like activity. J Clin Invest. 1978 Oct;62(4):892–895. doi: 10.1172/JCI109203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Lukas T. J., Hawiger J. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the gamma chain. Biochemistry. 1984 Apr 10;23(8):1767–1774. doi: 10.1021/bi00303a028. [DOI] [PubMed] [Google Scholar]
- Kolenich J. J., Mansour E. G., Flynn A. Haematological effects of aspirin. Lancet. 1972 Sep 30;2(7779):714–714. doi: 10.1016/s0140-6736(72)92124-1. [DOI] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- Lahav J., Hynes R. O. Involvement of fibronectin, Von Willebrand factor, and fibrinogen in platelet interaction with solid substrata. J Supramol Struct Cell Biochem. 1981;17(4):299–311. doi: 10.1002/jsscb.380170402. [DOI] [PubMed] [Google Scholar]
- Lerner W. A., Pearlstein E., Ambrogio C., Karpatkin S. A new mechanism for tumor induced platelet aggregation. Comparison with mechanisms shared by other tumor with possible pharmacologic strategy toward prevention of metastases. Int J Cancer. 1983 Apr 15;31(4):463–469. doi: 10.1002/ijc.2910310411. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Ambrogio C., Karpatkin S. Effect of antiplatelet antibody on the development of pulmonary metastases following injection of CT26 colon adenocarcinoma, Lewis lung carcinoma, and B16 amelanotic melanoma tumor cells into mice. Cancer Res. 1984 Sep;44(9):3884–3887. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Plow E. F., Srouji A. H., Meyer D., Marguerie G., Ginsberg M. H. Evidence that three adhesive proteins interact with a common recognition site on activated platelets. J Biol Chem. 1984 May 10;259(9):5388–5391. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Read M. S., Potter J. Y., Brinkhous K. M. Venom coagglutinin for detection of von Willebrand factor activity in animal plasmas. J Lab Clin Med. 1983 Jan;101(1):74–82. [PubMed] [Google Scholar]
- Ruggeri Z. M., Bader R., de Marco L. Glanzmann thrombasthenia: deficient binding of von Willebrand factor to thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Immunochemical and collagen-binding properties of fibronectin. Ann N Y Acad Sci. 1978 Jun 20;312:178–191. doi: 10.1111/j.1749-6632.1978.tb16802.x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 1984;3(1):43–51. doi: 10.1007/BF00047692. [DOI] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Varon D., Karpatkin S. A monoclonal anti-platelet antibody with decreased reactivity for autoimmune thrombocytopenic platelets. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6992–6995. doi: 10.1073/pnas.80.22.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S., Jr, Hilgard P. Aspirin and tumour metastasis. Lancet. 1972 Dec 30;2(7792):1416–1417. doi: 10.1016/s0140-6736(72)92982-0. [DOI] [PubMed] [Google Scholar]


