Table 1.
Catalytic Enantioselective Synthesis of 2-Vinylchromanes 1a–c.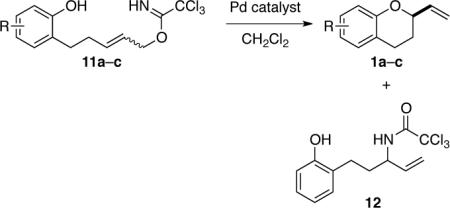
| entrya | substrate | R | catalyst (mol %) | temp (°C) | yield (%)b12 | yield (%)b1 | ee (%)c1 |
|---|---|---|---|---|---|---|---|
| 1 | (E)-11a | H | 7 (2) | 38 | — | 86 | 89 (R)d |
| 2 | (E)-11a | H | 10 (2) | 38 | — | 72 | 80 (S) |
| 3 | (E)-11a | H | ent-9 (2) | 38 | 43 | 41 | 80 (S) |
| 4 | (E)-11a | H | 8 (2) | 38 | 13 | 81 | 87 (R) |
| 5 | (E)-11a | H | 7 (0.5) | 23 | — | 91e | 94 (R)e |
| 6 | (E)-11a | H | ent-7 (0.5) | 23 | — | 97e | 92 (S)e |
| 7 | (E)-11b | 4-Br | 7 (0.5) | 23 | — | 96e | 80e |
| 8f | (E)-11b | 4-Br | 7 (0.5) | 23 | — | 94e | 90e |
| 9g | (E)-11c | 4-OMe | 7 (0.5) | 23 | — | 92e | 91 (R)d,e |
| 10 | (Z)-11a | H | ent-7 (0.5) | 23 | — | 92e | 9 (R)e |
[11] = 0.2 M; reaction time 8–18 h.
Isolated yield after purification on silica gel.
Determined by HPLC analysis using a enantioselective stationary phase.
Absolute configuration determined by comparison of optical rotation data with that reported in the literature.4b
Mean values of duplicate reactions.
The solvent was CHCl3.
Reaction time was 30 h.
