Figure 7.
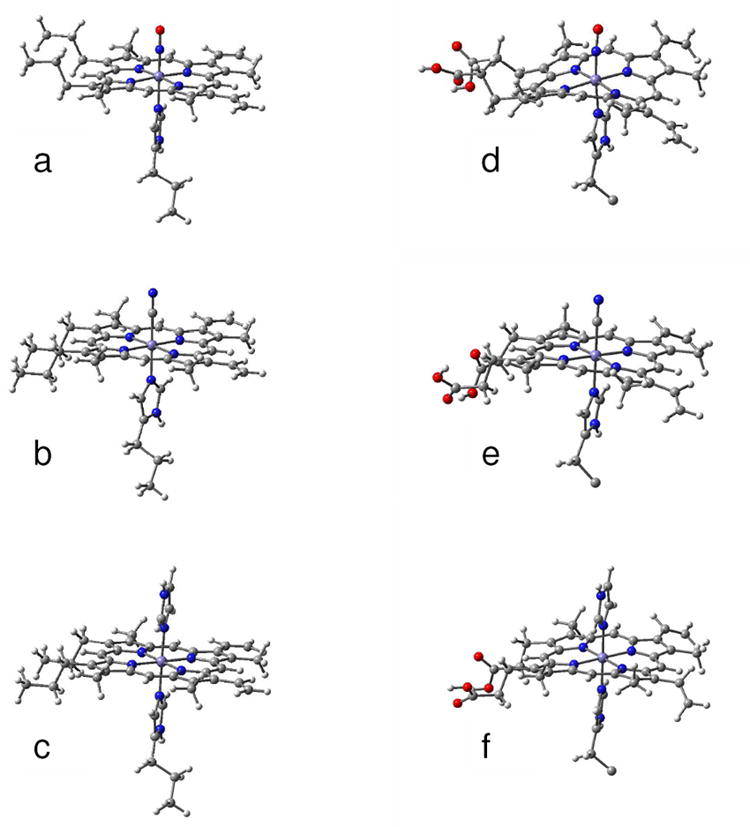
Optimized structures of the heme group and its ligand of (a) NP2–NO, (b) NP2–CN and (c) NP2-Hm which were obtained from DFT calculation on the shown molecular structures only. The functional groups of the heme and the histidine ligand were replaced by methyl groups. For comparison the optimized molecular structures calculated with the ONIOM method of (d) NP2–NO, (e) NP2–CN and (f) NP2-Hm are shown. Only the parts of the molecules treated with DFT are displayed. The residual parts of the protein, which were taken into account by force field calculations, are not shown.
