Abstract
Two hundred female sex workers (FSWs) in Lima, Peru were randomized to receive HPV4 vaccine in the standard (0, 2, 6 months) or a modified schedule (0, 3, 6 months). One hundred and eighty four (92%) participants completed 3 doses of vaccine. Baseline seropositive rates were 58% for HPV6, 22.5% for HPV11, 41.5% for HPV16, and 13% for HPV18. The final geometric mean antibody titer (GMT) following vaccination was significantly greater for women who were seropositive at baseline compared to seronegative women: HPV6 (GMT ratio=2.3, p<0.01), HPV11 (GMT ratio=2.7, p<0.01), HPV16 (GMT ratio=1.3, p=0.04), and HPV18 (GMT ratio=2.4, p<0.01)). Antibody titers in the modified schedule were not inferior to those in the standard schedule, suggesting the modified schedule may be paired with required STD visits. Although all women benefit from vaccination, administration at a younger age and before sexual debut is needed to achieve maximum protection from vaccine.
Keywords: Human Papillomavirus, HPV vaccine, adherence, immune response, female sex workers, Peru
Introduction
Approximately 500,000 women develop cervical cancer each year worldwide, and persistent human papillomavirus (HPV) infection is found in nearly all cases [1]. Studies of HPV vaccines were conducted in girls and young women 9–26 years of age with the primary objective to prevent cervical cancer [2]. HPV vaccines have been shown to be highly efficacious against cervical intraepithelial neoplasia associated with types 16 and 18 in women who were not infected at the time of immunization [3]. For each HPV4 associated genotype, antibody titer at 1 month following final vaccine dose was 27 to 145 times higher among placebo recipients who were seropositive at baseline [2].
Female sex workers (FSWs) are presumably at higher risk of HPV infection and cervical cancer than the general population due to their exposure to multiple sexual partners [4,5]. Studies of HPV among FSWs worldwide report cervical HPV DNA prevalence rates of 2.3% to 100% [6,10]. DNA prevalence of HPV4-associated genotypes among FSWs ranged from 3.4 to 45.8% in studies in Spain and Mexico [9,10]. We have identified one article which describes general HPV antibody prevalence among FSWs, but specific antibody values are not indicated [10].
HPV DNA prevalence among women in Peru is 17.7%, nearly twice the worldwide rate; cervical cancer is the leading cause of cancer death in Peruvian women, responsible for 20.6% of cancer deaths [11,12]. FSWs in Peru are required to receive STD and HIV testing every 3 months to obtain their health card and maintain their legal working status in brothels. Fewer than 10% of Peruvian FSWs were aware of HPV vaccine in previous studies [13].
Vaccination of new brothel-based FSWs at routine screening visits could increase completion rates, lower the risk of HPV related disease, and potentially decrease transmission to sex partners and clients [14]. We provided HPV vaccine to FSWs in Lima, Peru and collected serum before and after vaccination to evaluate the serologic response rates by baseline serologic status. We also investigated a modified immunization schedule and its effect on vaccine completion.
Materials and Methods
FSWs 18–26 years of age were recruited between August 28, 2009 and March 3, 2010 from 49 different sex locales in Lima, Peru by trained medical staff and 8 health promoters. Inclusion criteria were: registered FSW aged 18–26 years, living in Lima, no reported immune deficiency (including HIV), not pregnant or planning a pregnancy in the next 7 months, having a uterus, and not having received HPV vaccine. Participants were randomized in a 1 to 1 ratio to receive HPV4 vaccine in the standard (0, 2, 6 months) or a modified schedule (0, 3, 6 months) which paired more closely with 3 month clinic visits to receive STI testing. Stata 9.0 (Statacorp, College Station, TX) was used by study investigators to generate a random allocation sequence for the two study arms in block sizes of 8 to maintain balance in treatment groups. Participants opened sequentially numbered sealed envelopes with a letter written on paper which corresponded to study arms (0, 2, 6) or (0, 3, 6). All women were asked to return for their next visit according to their schedule, and to return for a final study visit one month after the third vaccine dose.
Baseline surveys consisted of 52 questions including demographic data, sexual health, condom use, HPV knowledge, barriers to vaccination, and medical history. Surveys were administered in Spanish by a trained interviewer. All participants had a physical examination. A cervical swab was collected for HPV DNA testing using the Digene HPV sampling kit (Qiagen). Five milliliters of blood was collected at baseline and one month following final vaccination dose.
Data analyses
Survey data and laboratory results were analyzed in EpiInfo 3.5.1 and Stata 10.0. Pearson’s chi-square tests were computed to test for differences in variables by baseline serostatus. The association between HPV DNA prevalence and serology was calculated using Fisher’s exact tests. Comparison of antibody titers was done using t-tests on log transformed data. Associations of variables with antibody response were calculated using linear regression on log transformed antibody titer and p-values are from F-testing. Adherence was measured as receiving all 3 vaccine doses within a 30 day window of the scheduled vaccine dose.
Sample size was calculated using PASS 2008. With 80% power, type 1 error of 0.05, standard deviations of 0.6, and an equivalence margin of 0.3, 64 women were needed per study arm to detect non-inferiority. The primary outcome was antibody response following vaccination in the two study arms. Secondary outcomes included seroprevalence prior to vaccination and a comparison of seropositivity to cervical HPV DNA prevalence.
Cervical samples
Cervical samples were aliquoted, refrigerated at −20° C, and sent to Johns Hopkins Bloomberg School of Public Health for testing. Aliquots of water without sampling were shipped from Peru and tested as negative controls. DNA was extracted using the QIAamp DNA Blood Kit (Qiagen). SiHa and K562 cells spiked into STM collection medium were used as positive and negative extraction controls, respectively in each extraction batch. Samples were analyzed for the presence or absence of HPV DNA and genotyped using the Roche HPV Linear Array test.
HPV antibody titer
Within 30 minutes of collection, blood was centrifuged at 20 degrees Celsius for 5 minutes at 3000 rpm. Serum was removed, stored in 2 aliquots, and shipped to Pharmaceutical Product Development (Wayne, PA) for testing using the multiplexed competitive Luminex immunoassay [15,16]. All values were reported in milli Merck units (mMu). The established antibody cutoffs for seropositivity of the HPV competitive Luminex immunoassay are: HPV6=20 mMu, HPV11=16 mMu, HPV16=20 mMu, HPV18=24 mMu as per the analysis by Dias et al [16].
Institutional review board (IRB) approval
This study was approved by IRBs at the Johns Hopkins Bloomberg School of Public Health in Baltimore, MD, and the Universidad Peruana Cayetano Heredia and Via Libre in Lima, Peru. All participants provided written informed consent. This clinical study was registered with clinicaltrials.gov identifier NCT00925288 under trial registry name “Acceptability of Human Papillomavirus (HPV) Vaccine in Female Sex Workers (Girasol)”.
Results
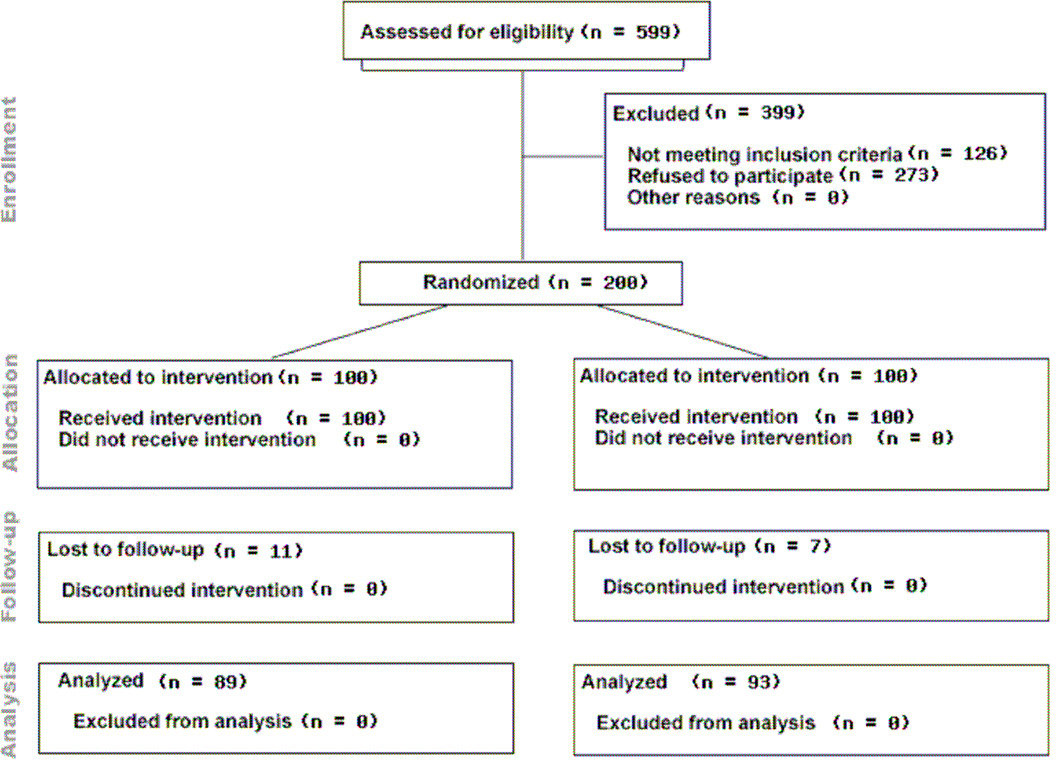
Five hundred and ninety nine women were screened for eligibility between August 28, 2009 and March 3, 2010; 399 women were excluded (n=126 non eligible, n=273 refused to participate). Two hundred participants were randomized to receive HPV vaccine in the standard or a modified schedule (Figure 1). One hundred and eighty four (92%) participants completed 3 doses of vaccine, and 95% of those adhered to the schedule, with the final vaccine visit blood draw on December 3, 2010. There were no differences in vaccine completion by study arm, with 91 participants completing the 0, 2, 6 schedule and 93 completing the 0, 3, 6 schedule. Eleven participants were lost to follow-up (LFU) before the final blood draw in the 0, 2, 6 schedule, compared to 7 participants LFU in the 0, 3, 6 schedule, and 182 samples were analyzed for HPV antibody titer. No adverse events were experienced or reported by participants.
Figure 1.
CONSORT Diagram for study
The average age of participants was 22.9 years. Mean age at first sex was 15.9 years. Seventy percent of women had previously heard of HPV. One hundred and fifty eight participants (79%) were seropositive for HPV6, 11, 16, or 18 at baseline. Presence of a HPV4 associated genotype (OR=4.90, 95% CI 1.43–16.71), never having ever heard of HPV (OR=0.42, 95%CI 0.21–0.86) and having a low or normal BMI (OR=0.87, 95%CI 0.76–0.98) were significantly associated with HPV4-associated seropositivity (Table 1). Having an STD in the past year (OR=2.18, 95%CI 0.91–5.26) and prevalence of any HPV DNA (OR=1.94, 95%CI 0.96–3.89) was marginally associated with baseline serostatus. Age was not associated with HPV seroprevalence (OR=1.26, 95%CI 0.64–2.51).
Table 1.
Factors associated with HPV4 associated seropositivity at baseline among 200 FSWs in Lima, Peru
| Variable | Baseline Seronegative N (%) |
Baseline seropositive N (%) |
P-value |
|---|---|---|---|
| Age* | 22.8 (22–23.6) | 22.9 (22.6–23.3) | 0.73 |
| Education | |||
| Primary | 6 (14.3) | 11 (7.0) | 0.18 |
| Secondary | 24 (57.1) | 84 (53.2) | |
| University/Technical | 12 (28.6) | 63 (39.9) | |
| BMI* | 24.4 (23.5–23.4) | 23.4 (23–23.8) | 0.03 |
| Ever heard of HPV | 23 (54.8) | 117 (74.1) | 0.02 |
| Marital status single | 23 (54.8) | 98 (62.0) | 0.39 |
| HPV4 DNA positive | 3 (7.1) | 43 (27.4) | <0.01 |
| STD in past year | 7 (16.7) | 48 (30.4) | 0.08 |
| Vaginal discharge | 20 (47.6) | 61 (38.6) | 0.29 |
| Current genital warts | 1 (2.4) | 12 (7.6) | 0.31 |
| Age of first sex under 18 years | 36 (85.7) | 118 (74.7) | 0.15 |
| Years of sex* | 7.1 (6.2–7.9) | 6.9 (6.5–7.4) | 0.79 |
| Clients in past week* | 50.7 (16.6–84.9) | 40.9 (33.7–48.2) | 0.38 |
| Has non-paying partners | 28 (66.7) | 118 (74.7) | 0.30 |
| Number non-paying partners* | 0.9 (0.9–1.0) | 1.0 (1.0–1.1) | 0.15 |
| Any condom use with partners | 25 (59.5) | 86 (54.4) | 0.56 |
mean (95%CI) computed
Baseline seropositive rates were 58% for HPV6, 22.5% for HPV11, 41.5% for HPV16 and 13% for HPV18. Four women (2%) were seropositive for all 4 genotypes, 31 (15.5%) had both types 6 and 11, and 12 (6%) women were seropositive for types 16 and 18. One hundred and fifty eight women were seropositive for any HPV4 type. In total, 11 women had genital warts at baseline, of which 7 were seropositive for HPV6. Baseline geometric mean antibody titers did not vary by study schedule (p>0.2 for all HPV4 genotypes).
Comparison of HPV DNA and baseline seropositivity
We have cervical HPV DNA results from 199 participants and serology from 200 participants. Twenty three percent of participants were DNA positive for any of the HPV4-associated genotypes at baseline, compared to 79% who were seropositive for the same types (Table 2). No participants had more than one HPV4 genotype in DNA testing, while 42% of participants were seropositive for at least two HPV4 types, and 12% were seropositive for three HPV4 types.
Table 2.
Baseline HPV DNA and antibody status by HPV type among 199 FSWs in Lima, Peru
| HPV prevalence | Antibody positive | Antibody negative | ||||
|---|---|---|---|---|---|---|
| DNA | Antibody | DNA− | DNA+ | DNA− | DNA+ | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| HPV6 | 11 (5.5) | 116 (58.3) | 109 (54.8) | 7 (3.5) | 79 (39.7) | 4 (2.0) |
| HPV11 | 1 (0.5) | 45 (22.6) | 45 (22.6) | 0 (0) | 153 (76.9) | 1 (0.5) |
| HPV16 | 28 (14.7) | 82 (41.2) | 63 (31.7) | 19 (9.5) | 108 (54.3) | 9 (4.5) |
| HPV18 | 6 (3.0) | 26 (13.1) | 26 (13.1) | 0 (0) | 167 (83.9) | 6 (3.0) |
| Any HPV4 | 46 (23.1) | 157 (78.9) | 114 (57.3) | 43 (21.6) | 39 (19.6) | 3 (1.5) |
Significantly more women were seropositive for HPV16 (OR=3.6, 95%CI 1.54–8.48) and for any HPV4-associated genotype (OR=4.9, 95%CI 1.43–16.7) than DNA positive. There were no significant differences in HPV6, 11, or 18 alone in DNA and sera although differences in DNA and sera for types 6 and 11, which cause genital warts, are much greater than for 16 and 18. More than 93% of women positive on DNA testing for an HPV4 genotype were seropositive for that type (p<0.01). The baseline HPV16 GMT was significantly higher among women who were HPV DNA-positive (p<0.01).
Antibody response to vaccine
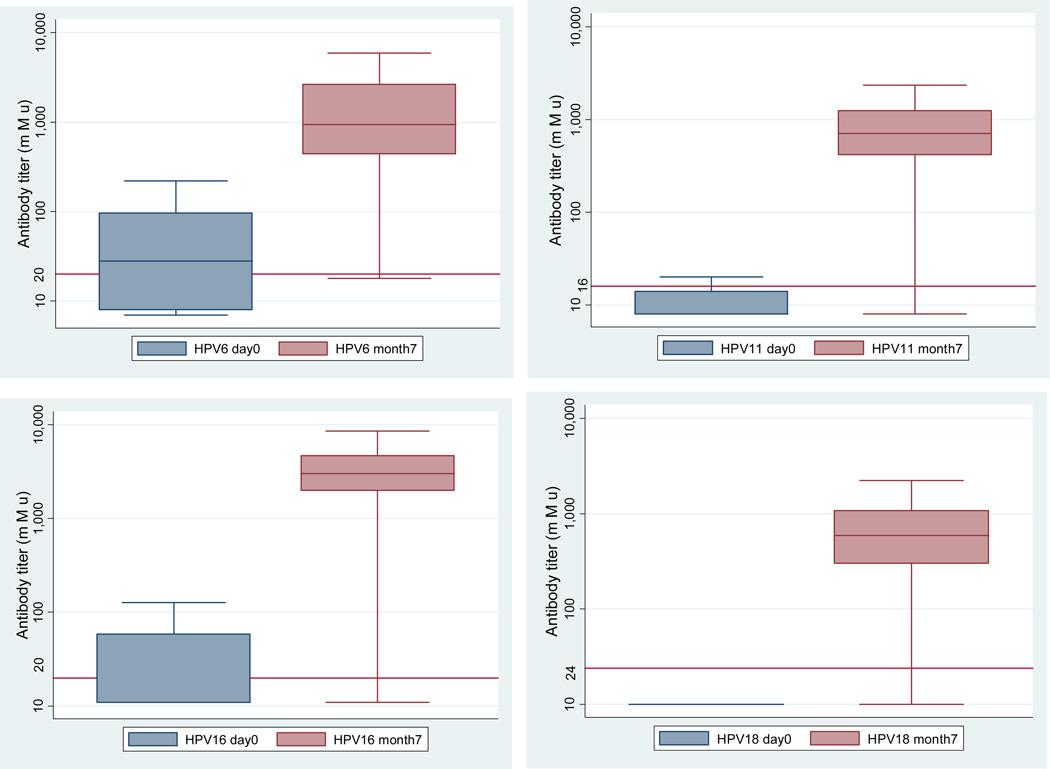
Nearly all (99.5%) of the 182 women whose blood was tested one month after last vaccination responded to each of the 4 vaccine types regardless of baseline serostatus. The final GMTs after vaccination were 1069.8 for HPV6, 761.1 for HPV11, 2952.5 for HPV16, and 565.6 for HPV18 (Figure 2). The mean fold increase in GMT from baseline to 1 month after final vaccine dose was 65.6 for HPV6, 91.2 for HPV11, 214.1 for HPV16, and 64.4 for HPV18, with a significantly greater increase among baseline seronegative women (all p<0.01). Among baseline seropositive women, the mean fold increase in GMT from baseline to 1 month after final vaccine dose was 17.1 for HPV6, 32.0 for HPV11, 35.6 for HPV16, and 16.8 for HPV18.
Figure 2.
Antibody levels at baseline and 1 month following final vaccine dose by genotype among 182 FSWs in Lima, Peru.
Horizontal lines represent antibody cutoffs for seropositivity
The box contains 25th to 75th percentile, and the upper bars are 95%
Statistically significant differences between antibody levels at day0/month7 P<.01
There were no significant differences in the final GMTs of antibody by study arm for any HPV4 type (p>0.2). In the intention to treat (ITT) analysis, the final antibody concentrations following vaccination were significantly higher for women who were seropositive at baseline compared to women who were seronegative for all HPV4 types; HPV6 (GMT ratio=2.3), HPV11 (GMT ratio=2.7), HPV16 (GMT ratio=1.3), and HPV18 (GMT ratio=2.4) (Table 3). Excluding participants who did not adhere to the study schedule did not change the results.
Table 3.
Geometric mean titer and 95% confidence for month 7 HPV antibody levels of 182 FSWs by baseline serostatus
| Total | Baseline Seronegative | Baseline Seropositive | p-value* | ||||
|---|---|---|---|---|---|---|---|
| N | GMT (95%CI) | N | GMT (95%CI) | N | GMT (95%CI) | ||
| HPV6 | 182 | 1069.8 (895.5–1278.1) | 77 | 658 (536.3–807.3) | 105 | 1528 (1189.3–1963.1) | <0.01 |
| HPV11 | 182 | 761.1 (656.4–882.6) | 141 | 606.7 (535–688) | 41 | 1660.3 (1082.7–2546.1) | <0.01 |
| HPV16 | 182 | 2952.5 (2604–3347.7) | 105 | 2641.5 (2211–3156) | 77 | 3436.4 (2900–4072.4) | 0.04 |
| HPV18 | 182 | 565.6 (491.9–650.4) | 158 | 504.6 (437.5–582) | 24 | 1198.3 (808.4–1776.4) | <0.01 |
t-test on log titers
All GMT values are in mMu
Women who were PCR-negative at baseline had a higher GMT antibody response to vaccine compared to PCR positives, although this was not statistically significant (Table 4). Participants who were baseline seropositive had a higher GMT antibody response to vaccine compared to baseline seronegatives (all p<0.05). Adherence to the vaccine schedule was associated with a higher month 7 antibody titer for HPV6 (p=0.03), and a marginally higher titer for HPV11 (p=0.06) and HPV16 (p=0.07) compared to non-adherence. In addition, the number of weeks lapsed from the final vaccine dose until the final blood draw was a significant predictor of final antibody response for HPV types 11, 16, and 18.
Table 4.
Univariate linear regression analysis of factors associated with month 7 antibody response among 182 FSWs in Lima, Peru
| HPV6 | HPV11 | HPV16 | HPV18 | |||||
|---|---|---|---|---|---|---|---|---|
| B1 | P-value | B1 | P-value | B1 | P-value | B1 | P-value | |
| Day 0 genotype seropositivity | 0.84 | <0.01 | 1.00 | <0.01 | 0.26 | 0.04 | 0.86 | <0.01 |
| Day 0 DNA positive | −0.60 | 0.1 | −0.14 | 0.89 | −0.11 | 0.54 | −0.33 | 0.45 |
| Nationality (Peruvian vs other) | −0.69 | <0.01 | −0.04 | 0.85 | 0.08 | 0.65 | −0.11 | 0.57 |
| Study arm (026 vs 036) | −0.01 | 0.87 | 0.01 | 0.78 | −0.18 | 0.16 | −0.01 | 0.37 |
| BMI (normal weight vs not) | 0.25 | 0.19 | 0.32 | 0.05 | 0.14 | 0.3 | 0.23 | 0.13 |
| Vaginal discharge present | −0.30 | 0.1 | −0.03 | 0.85 | 0.21 | 0.87 | −0.28 | 0.05 |
| In window dose 3- final blood | 1.15 | 0.02 | 0.69 | 0.1 | 0.43 | 0.23 | 0.83 | 0.04 |
| In all study windows | 0.66 | 0.03 | 0.48 | 0.06 | 0.39 | 0.08 | 0.30 | 0.22 |
| Number weeks dose 3-final blood | −0.13 | 0.16 | −0.21 | <0.01 | −0.17 | 0.01 | −0.24 | <0.01 |
=Beta coefficients from linear regression of log transformed month 7 serotype specific antibody titer
Discussion
FSWs in Lima, Peru had a high seroprevalence of HPV4 associated genotypes, and almost all tested positive following vaccination. Antibody titers in the modified schedule were not inferior to those in the standard schedule, suggesting the modified schedule may be paired with required STD visits. High baseline HPV seroprevalence compared to DNA prevalence in this study suggests that DNA is a poor predictor of prior exposure to HPV in FSWs, as many women with antibody responses from prior infections have cleared the viruses and are not currently infected with the virus. To our knowledge, this is the first study which compares HPV DNA prevalence to seroprevalence in FSWs, and antibody response following vaccination among a majority of baseline seropositive persons.
Although it is recommended to receive HPV vaccines prior to sexual debut, a recent study has shown that HPV vaccines induce a robust and persistent immune response in women over 26 years of age [17]. Our results show that 98% of study participants did not have antibody against all HPV4 associated genotypes prior to vaccination, and thus may have benefited in some way from vaccination. HPV antibody prevalence identified in this study is comparable to a previous study in FSWs in Spain which showed a 45.8% prevalence for HPV16 and 23.2% for HPV18 [10]. In fact, the GMT of antibody was 17–36 fold higher one month after 3 vaccine doses among baseline seropositive women, as they were immunologically primed prior to receiving vaccine. In addition, a recent study of 2617 women suggests that naturally acquired HPV antibodies may not provide complete protection from reinfection or reactivation over time [18].
Antibody titers did not vary significantly by study schedule. There were no significant differences in vaccine completion rates by study schedule (0, 2, 6 vs 0, 3, 6), suggesting that vaccination can occur at the time of required STD clinic visits. Longer (e.g. yearly) intervals would have likely resulted in lower completion rates due to high movement of this population. However, this is not a justification for not starting the immunization series as yearly intervals for hepatitis B vaccine have been shown to be highly effective [19]. Varying vaccine intervals for hepatitis B vaccine in FSWs in Belgium did not affect the immune response to the vaccine [20]. Similar results have been shown in two other studies of HPV vaccine, with non-inferior GMT for vaccine administration at yearly and biannual intervals, as well as at 0,2, and 12 months [21,22] One woman in our study did not mount an immune response to any of the four HPV serotypes, although she received all 3 vaccine doses. After confirming that all specimens were properly tested and samples were handled properly, we believe that she may have had a primary immunodeficiency, but we have not been able to conduct a follow-up evaluation.
There are several limitations in our study. We do not have data on time of infection with HPV, and thus cannot distinguish between antibodies resulting from vaccination and antibodies resulting from natural HPV infection during the 6 month vaccination period. In addition, we did not collect data on smoking status of participants. Smoking has been shown to be associated with low grade squamous intraepithelial lesions and decreased antibody response to some vaccines [23]. A previous study of 319 FSWs in Lima showed that 45.5% were current smokers and 46.4% drank alcohol before work [13].
Our results are not generalizable to all Peruvian FSWs. Women who participated in our study may have been more aware of the consequences of unsafe sex and used condoms more frequently with their clients compared to non-participants. There are an estimated 88,640 FSWs in Peru, and 18,000 in Lima [4,24].
Conclusions
We achieved high HPV vaccine completion rates in a sample of FSWs in Lima, Peru. Substantial recruitment and retention efforts may be necessary to achieve similar results in FSWs or other high risk groups in other countries, and at a larger scale. Altering the vaccination schedule did not change the immune response to vaccine, and immunization is more convenient if paired with required STD visits. A two-dose and shorter schedule may be easier to complete for hard-to-reach populations, and studies are underway to evaluate antibody responses and protection after 2 doses.
Nearly all women mounted an immune response to vaccine; women primed by natural HPV infection prior to vaccination had higher antibody levels after vaccination than baseline seronegative women. Women who were not infected before vaccination may have received protection from the relevant vaccine genotypes, and women who were already infected may have received protection from reinfection or reactivation following antibody increases with vaccine.
The high seroprevalence of HPV4 associated genotypes at baseline highlights the need to vaccinate women before sexual debut to attain maximum protection. FSWs who are vaccinated early on in their sexual careers may also receive significant protection against chronic infection by HPV4 genotypes. Cost effectiveness data and potential number of cancers averted are needed before consideration of HPV4 vaccine in FSWs.
Highlights.
Female sex workers in Peru were randomized to receive HPV4 in two study schedules.
More women were seropositive for any HPV4 genotype at baseline than DNA positive.
We achieved high vaccine completion, and nearly all women had an immune response.
Antibody titers in the modified schedule were not inferior to the standard schedule.
Vaccination before sexual debut is needed to achieve maximum protection.
Acknowledgements
The authors would like to thank study team members Lisbeth de la Rosa, Cristina Esteves, Gita Byraiah, and Ylda Lopez for their assistance in data collection. We would also like to thank the brothel managers and health promoters for their efforts. Thanks to Rhonda Heffelfinger-Wenner and Katie Matys for their technical assistance in HPV antibody analysis. Thanks to Yolanda Eby and Roslyn Howard for their technical assistance in HPV genotyping. Tina Proveaux provided technical and editorial assistance.
Funding: Supported in part by a research grant from the Merck Investigator-Initiated Studies Program of Merck & Co., Inc. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck & Co., Inc. This research was funded, in part, by the Department of International Health Global Disease Epidemiology Program, Delta Omega Scholarship, Dan David Prize Scholarship, Carol Eliasberg Martin Scholarship in Cancer Prevention, NIH Postdoctoral training grant # T32 MH080634, NIH Predoctoral National Research Service Award F31AI080187, Fogarty International Clinical (FIC) Research Fellows Program, and FIC/NIH grant 1R01TW008398.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: Neal Halsey received financial compensation for serving on Safety Monitoring Boards for the post-licensure safety assessment evaluation of Gardasil and other vaccines within the past two years. He received no financial support for participation in this study. Other authors: None declared.
Contributors: BB created the study materials, carried out the data cleaning and data analysis, supervised all study activities, and wrote the first draft of the text. NH is the Principal Investigator of the study, provided guidance in all aspects of the study, and helped to obtain funding; CC is the local Principal Investigator of the study and provided guidance during the study; MB is the local Co-Investigator, collaborated in the elaboration of study materials, training of health personnel, and helped complete the IRB processes in Peru. AC is the local study coordinator, trained the team in sample collection, helped translate all materials, and processed the samples. PG is the Co-Investigator and conducted the reported HPV DNA testing; all authors contributed to the final manuscript.
References
- 1.IARC. Monographs on the evaluation of carcinogenic risks to humans: human papillomaviruses. Vol 90. Lyon: International Agency for Research on Cancer; 2007. Feb 15–22, [PMC free article] [PubMed] [Google Scholar]
- 2.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6 11 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 3.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calhoun B. Feasibility of a Community-based Voluntary HIV Counseling and Testing Program in Commercial Sex Venues in Lima, Peru; Third Annual Poster Presentation at Puget Sound Partners for Global Health Lecture; Puget Sound, Washington: 2005. [Google Scholar]
- 5.Mak R, Van Renterghem L, Cuvelier C. Cervical smears and human papillomavirus typing in sex workers. Sex Transm Infect. 2007;80:118–120. doi: 10.1136/sti.2002.003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donmez H, Menevse S, Guner H, Menevse A. Detection and typing of human papillomavirus DNAs by restriction endonuclease mapping of the PCR products. Isr J Med Sci. 1997;33:789–793. [PubMed] [Google Scholar]
- 7.Volkow P, Rubi S, Lizano M, Carrillo A, Vilar-Compte D, Garcia-Carranca A, et al. High prevalence of oncogenic human papillomavirus in the genital tract of women with human immunodeficiency virus. Gynecol Oncol. 2001;82:27–31. doi: 10.1006/gyno.2001.6244. [DOI] [PubMed] [Google Scholar]
- 8.Marais D, Carrara H, Kay P, Ramjee G, Allan B, Williamson AL. The impact of the use of COL-1492, a nonoxynol-9 vaginal gel on the presence of cervical human papillomavirus in female sex workers. Virus Res. 2006;121:220–222. doi: 10.1016/j.virusres.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Juárez-Figueroa LA, Wheeler CM, Uribe-Salas FJ, Conde-Glez CJ, Zampilpa-Mejía LG, García-Cisneros S, et al. Human Papillomavirus: A Highly Prevalent Sexually Transmitted Disease Agent Among Female Sex Workers From Mexico City. Sex Transm Dis. 2001;28:125–130. doi: 10.1097/00007435-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Touze A, de Sanjose S, Coursaget P, Almirall MR, Palacio V, Meijer CJ, et al. Prevalence of anti-human papillomavirus type 16, 18, 31, and 58 virus-like particles in women in the general population and in prostitutes. J Clin Microbiol. 2001;39:4344–4348. doi: 10.1128/JCM.39.12.4344-4348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 12.Ferlay J, Bray F, Pisani P, Parkin DM. IARC. 2004. Globocan 2002: Cancer Incidence, Mortality, and Prevalence Worldwide. [Google Scholar]
- 13.Brown B, Carcamo C, Blas MM, Valderrama M, Halsey N. Peruvian FSWs: Understanding HPV and barriers to vaccination. Vaccine. 28(49):7743–7747. doi: 10.1016/j.vaccine.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 14.Munoz N, Manalastas R, Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6 11 16 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 15.Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, et al. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6,11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10:108–115. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clinical and diagnostic laboratory immunology. 2005;12:959–969. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15–55 years. Vaccine. 2009;27:581–587. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 18.Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Evaluation of quadrivalent HPV6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Human vaccines. 2009;5(10):696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 19.Halsey NA, Moulton LH, O'Donovan JC, Walcher JR, Thoms ML, Margolis HS, et al. Hepatitis B vaccine administered to children and adolescents at yearly intervals. Pediatrics. 1999;103:1243–1247. doi: 10.1542/peds.103.6.1243. [DOI] [PubMed] [Google Scholar]
- 20.Wouters K, Leuridan E, Van Herck K, Van Ardenne N, Roelofs I, Mak R, et al. Compliance and immunogenicity of two hepatitis B vaccination schedules in sex workers in Belgium. Vaccine. 2007;25:1893–1900. doi: 10.1016/j.vaccine.2006.09.073. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman RK, Nowalk MP, Lin CJ, Fox DE, Ko FS, Wettick E, et al. Randomized trial of an alternate human papillomavirus vaccine administration schedule in college-aged women. J Womens Health (Larchmt) 2010;19(8):1441–1447. doi: 10.1089/jwh.2009.1753. [DOI] [PubMed] [Google Scholar]
- 22.Neuzil KM, Canh do G, Thiem VD, Janmohamed A, Huong VM, Tang Y, et al. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA. 2011;305(14):1424–1431. doi: 10.1001/jama.2011.407. [DOI] [PubMed] [Google Scholar]
- 23.Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 24.Vandepitte J, Lyerla R, Dallabetta G, Crabbe F, Alary M, Buve A. Estimates of the number of female sex workers in different regions of the world. Sex Transm Infect. 2006;82(Suppl 3):iii18–iii25. doi: 10.1136/sti.2006.020081. [DOI] [PMC free article] [PubMed] [Google Scholar]