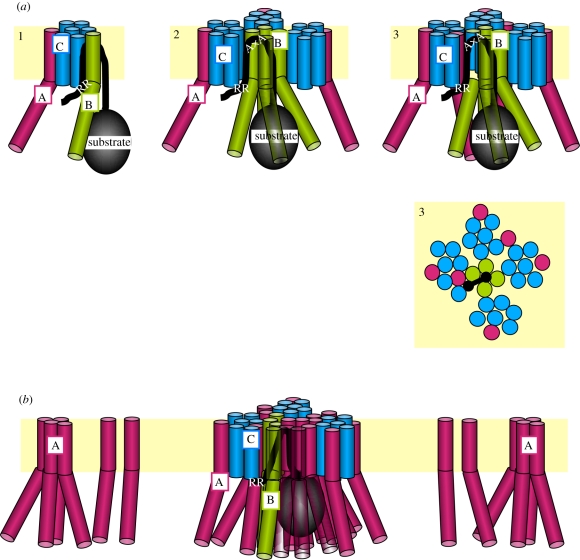
Figure 3.
Model of a substrate-induced formation of a functional Tat translocase. (a) Step 1: superficial binding of a Tat signal sequence to the Tat translocase involves the RR-consensus motif and cytosolic parts of TatC. The coordinate recognition of a Tat signal sequence by TatC and TatB leads to its hairpin-like insertion into the plane of the membrane. Step 2: TatB homo-oligomerizes and encapsulates the folded mature domain of the substrate. Step 3: a proton motive-force-dependent step brings monomeric TatA in close contact to the signal sequence. AxA denotes the C-terminal end of the signal sequence. Step 3 is also shown as top view from the periplasmic side of the membrane. (b) More TatA subunits are recruited from a pool of monomeric TatA or more likely in tetrameric units. Processive oligomerization leads to the pavement of a transmembrane path. This might comprise oligomeric TatA pores, hetero-oligomeric TatA(BC) pores as drawn here or some other undefined TatA structure.