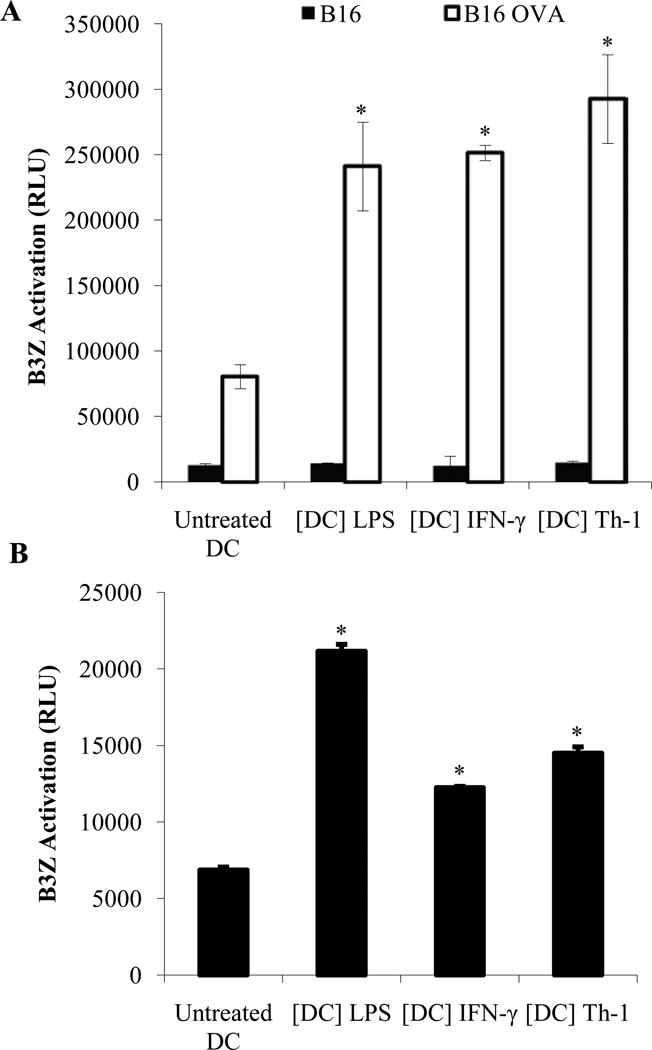
Figure 7. Th-1 KDCs are capable of presenting tumor antigens from the tumor cells they have killed to tumor-specific T cells.
CD11c+ DCs were treated with LPS (1 µg/ml) ([DC] LPS), IFN-γ (5 ng/ml) ([DC] IFN-γ) or Th-1 supernatant ([DC] Th-1) for 48 hours. (A) DCs were then washed and cultured for 24 hours with B16 or B16-OVA melanoma cells. DCs were selected from the culture using CD11c microbeads and incubated for an additional 24 hours with B3Z cells (DC:B3Z ratio = 1:10) as outlined in material and methods. The activity of β-galactosidase was measured by evaluating the conversion of its substrate into a chemoluminescent product (RLU, Relative Luminescence Unit). (B) CD11c-GFP-DTR mice were injected with B16-OVA melanoma cells. When tumors become palpable, diphtheria toxin (DT) was administered to deplete host CD11c+ cells. CD11c+ DCs generated in vitro and treated with LPS or IFN-γ or Th-1 supernatant were then injected intratumorally (20×106 cells/tumor). After 36 hours, draining lymph nodes were harvested and CD11c+ cells were isolated using CD11c microbeads. The obtained DCs were then cultured with the OVA-specific T cell line B3Z. Specific recognition of tumor-derived OVA peptide was measured as outlined above. * Significant difference when compared to untreated DC group (p<0.01).