Abstract
During mouse embryogenesis, proper formation of the heart and liver is especially important and is crucial for embryonic viability. In this study, we showed that Mab21l2 was expressed in the trabecular and compact myocardium, and that deletion of Mab21l2 resulted in a reduction of the trabecular myocardium and thinning of the compact myocardium. Mab21l2-deficient embryonic hearts had decreased expression of genes that regulate cell proliferation and apoptosis of cardiomyocytes. These results show that Mab21l2 functions during heart development by regulating the expression of such genes. Mab21l2 was also expressed in the septum transversum mesenchyme (STM). Epicardial progenitor cells are localized to the anterior surface of the STM (proepicardium), and proepicardial cells migrate onto the surface of the heart and form the epicardium, which plays an important role in heart development. The rest of the STM is essential for the growth and survival of hepatoblasts, which are bipotential progenitors for hepatocytes and cholangiocytes. Proepicardial cells in Mab21l2-deficient embryos had defects in cell proliferation, which led to a small proepicardium, in which α4 integrin expression, which is essential for the migration of proepicardial cells, was down-regulated, suggesting that defects occurred in its migration. In Mab21l2-deficient embryos, epicardial formation was defective, suggesting that Mab21l2 plays important roles in epicardial formation through the regulation of the cell proliferation of proepicardial cells and the migratory process of proepicardial cells. Mab21l2-deficient embryos also exhibited hypoplasia of the STM surrounding hepatoblasts and decreased hepatoblast proliferation with a resultant loss of defective morphogenesis of the liver. These findings demonstrate that Mab21l2 plays a crucial role in both heart and liver development through STM formation.
Introduction
Two distinct heart fields, the FHF (first heart field) and the SHF (second heart field), are derived from anterior lateral plate mesoderm and splanchnic mesoderm, respectively. In the mouse embryo, the FHF forms a crescent shape at E7.5, and the cells coalesce along the ventral midline to form an early heart tube at E8.0. At this stage, the SHF begins to migrate to the anterior and posterior ends of the heart tube to form the right ventricle, conotruncus and part of the atria. The heart tube then undergoes a process involving rightward looping and expansion of the myocardium, leading to the formation of the four-chambered heart [1], [2]. Ventricular myocardial differentiation and maturation are characterized by the formation of both the compact myocardium and the trabecular myocardium. The compact myocardium forms in the outer zone of the ventricular wall and trabecular myocardium forms as protrusions along the inside of the compact myocardium [3]. Proper development of the compact and the trabecular myocardium is required for normal contractility and circulation of blood, and the importance of the appropriate development of these structures is demonstrated by the fact that deletion of genes essential for the normal development of these structures causes embryonic lethality at approximately midgestation.
Mammalian liver development begins with hepatic induction from the ventral endoderm through signaling molecules such as FGFs and BMPs, derived from the cardiac mesoderm and septum transversum mesenchyme (STM), respectively. After specification of the hepatic endoderm, the tissue starts extending towards the midgut. Following formation of the liver bud, the basement membrane surrounding it is progressively disrupted, and hepatoblasts (bipotential progenitors for hepatocytes and cholangiocytes) delaminate from the bud and invade the surrounding STM as cords. At this stage, the STM functions as a source of signals for hepatoblast growth and survival, and inactivation of genes encoding those signaling molecules causes liver hypoplasia (reviewed in [4], [5]).
The STM is required not only for liver development but also for heart development in forming the proepicardium. The proepicardium is a region of the STM caudal to the heart, and cells in this mesenchymal structure spread over the surface of the myocardium to form the epicardium, the epithelial outer layer of the heart [6], [7]. Epicardial cells undergo an epithelial-to-mesenchymal transition, penetrate into the neighboring myocardium and differentiate into the cells required for cardiovascular development, such as myofibroblast, pericytes, and smooth muscle cell [8], [9], [10], [11]. Epicardial cells are important not only as a source of cells contributing to heart development, but also as a source of signaling molecules regulating proliferation of the myocardium. Signaling molecules including erythropoietin, retinoic acid and FGF are produced in the epicardium, and communication between the epicardium and myocardium through these molecules is required for normal myocardial proliferation [12], [13]. Therefore, the STM is an important tissue involved in both heart and liver development.
The mab-21 gene was originally identified as a cell fate determinant in Caenorhabditis elegans and several studies have shown that mab-21 is highly conserved across animal species, from vertebrates to invertebrates [14], [15], [16]. Two mab-21 orthologues, Mab21l1 and Mab21l2, have been found in species such as zebrafish, Xenopus, chicken, mouse, and human [14], [16], [17], [18], [19], [20]. MAB-21 family proteins in vertebrates have more than 90% amino acid sequence similarity, but do not have any known functional motifs. Thus predicting the molecular functions of MAB-21 proteins from their amino acid sequence is difficult. Several studies in vertebrates have shown that Mab21l1 and Mab21l2 expression overlap in the eye, midbrain, branchial arches, and limb buds during development. In mouse, Mab21l1 is uniquely expressed in the lens and genital tubercle while Mab21l2 expression is found in the retina, body wall and umbilical cord [14], [16], [19], [20], [21], [22].
We have previously shown that Mab21l1-deficient mice have defects in eye and preputial gland development, and that MAB21L1 functions cell-autonomously in the lens. Additionally, Mab21l2-deficient mice, which are embryonic lethal between E11.5 and E14.5 (lethality rate of homozygous mutant embryos: E11.5, 14%; E12.5, 65%; E13.5, 64%), have defects in eye and body wall formation. Thus, Mab21l1 and Mab21l2 function during eye development; Mab21l1 is essential for lens placode development and Mab21l2 is essential for retina development. Mab21l1 and Mab21l2 are thought to function downstream of Pax6 in eye development [21], [23]. Mab21l1 is also essential for the appropriate development of the preputial glands, while Mab21l2 plays a crucial role in ventral body wall formation [21], [22].
We analyzed in detail, the expression of Mab21l2 and embryonic tissues of Mab21l2-deficient mice. Mab21l2 begins to be expressed in the heart region at approximately E8.5, and is expressed in both the trabecular and the compact myocardium at E9.5–E11.5, and deletion of Mab21l2 results in a reduction in the amount of trabecular myocardium and a thinning of the compact myocardium. BrdU and TUNEL assays demonstrated that cell proliferation decreased and apoptosis increased in Mab21l2 mutants. The Mab21l2 transcript is also found in the STM from the region of the proepicardium to the region near the hepatoblasts at E9.5, and Mab21l2 deletion causes defective morphogenesis of that tissue and results in defects in the epicardium and liver, demonstrating that Mab21l2 is essential for heart and liver development through the STM. Taken together, these results indicate that Mab21l2 is expressed in the cardiac field and the STM and plays essential roles during heart and liver development.
Results
Mab21l2 is expressed in the trabecular and compact myocardium and is required for normal development of these tissues
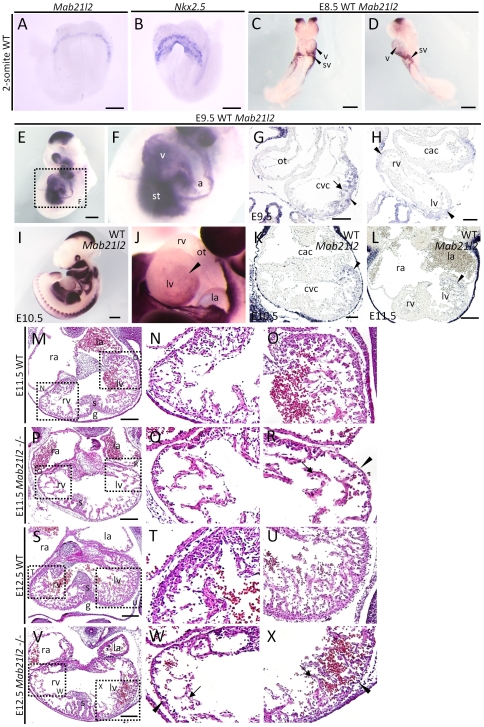
Embryonic lethality in Mab21l2-deficient embryos first occurred at approximately E11.5. Living embryos were observed until E13.5 [22], but in E13.5 Mab21l2 mutant embryos, the heartbeat was very weak. A known defect which results in embryonic lethality at around E11.5 is defective morphogenesis of the heart and cardiovascular system. Therefore, we examined whether the expression of Mab21l2 was detected in those tissues before E11.5 in wild-type embryos by in situ hybridization. At the 2-somite stage, Mab21l2 appeared to be expressed adjacent to the first heart field (identified by Nkx2.5 expression, the first heart field marker), i.e. the presumptive STM [24] (Figure 1A, B). At E8.5, the expression of Mab21l2 was detected in the ventricle and sinus venosus myocardium (Figure 1C, D). At E9.5, Mab21l2 was expressed in the STM (Figure 1E, F) and the ventricular myocardium, in both the trabecular and the compact myocardium (Figure 1G, S1A, B; Nkx2.5, myocardial marker). Especially, the expression was detected in the future right ventricle and left ventricle (Figure 1H black arrowheads). At E10.5 and E11.5, the expression of Mab21l2 was largely detected in the myocardium of the left ventricle, especially in the left side of the structures. The expression was strongest in the region between the atrium and ventricle (Figure 1I–L). At E12.5, the expression of Mab21l2 was only detectable at low levels in the trabecular region of the left ventricle (Figure S2A, B). The expression of Mab21l1, another mab-21 orthologue, was not detected in the heart region.
Figure 1. Mab21l2 is required for the formation of the trabecular and compact myocardium.
(A–F) Whole-mount in situ hybridization of 2-somite, E8.5 and E9.5 wild-type (WT) embryos for the indicated transcripts. (A, B) At the 2-somite stage, Mab21l2 appeared to be expressed adjacent to the first heart field expressing Nkx2.5. (C and D) Expression of Mab21l2 was detected in the ventricle, and sinus venosus at E8.5. (E and F) Mab21l2 was expressed in the ventricle (v) and the septum transversum mesenchyme (st) at E9.5. Scale bar represents 60 µm(A, B) and 300 µm (C–E). (G and H) In situ hybridization analysis of Mab21l2 in transverse paraffin sections of E9.5 WT embryonic heart. (G) Mab21l2 is expressed in the trabecular myocardium (arrow) and the compact myocardium (arrowhead). (H) Mab21l2 is expressed in the right and left ventricle (black arrowheads). Scale bars represent 50 µm (G) and 30 µm (H). (I and J) Whole-mount in situ hybridization of E10.5 WT embryos for Mab21l2. (J) Lateral view of the heart shown in (I). Scale bar in I represents 500 µm. (K and L) In situ hybridization analysis for Mab21l2 in transverse paraffin sections of E10.5 (K) and E11.5 (L) WT embryonic hearts. (K and L) Expression of Mab21l2 is seen on the left side of the left ventricle (arrowheads). Scale bars represent 50 µm in (K) and 100 µm (L). (M–X) Hematoxylin and eosin (H&E)-stained transverse sections of WT and Mab21l2-mutant embryonic hearts at E11.5 and E12.5. At E11.5, Mab21l2 mutant embryos show defects in the left ventricle (R), but not in the right ventricle (Q), including reduced trabecular myocardium (arrow) and thin compact myocardium (arrowheads) compared with WT embryos (N and O). Defects were observed in the right (W) and left (X) ventricle in Mab21l2-mutants compared with WT embryos at E12.5 (T and U) (compact myocardium, arrowheads; trabecular myocardium, arrows). a, atrium; cac, common atrium chamber; cvc, common ventricular chamber; ot, outflow tract; g, ventricular groove; la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle; v, ventricle; s, interventricular septum; st, septum transversum mesenchyme; sv, sinus venosus. Scale bar represents 100 µm (M, P, S and V).
To study the contribution of Mab21l2 expression to heart development, we examined hematoxylin and eosin (H&E) stained sections of Mab21l2-deficient embryos, and found defects in the heart region. In some Mab21l2 embryos (3/32 embryos) at E10.5, the compact myocardium of the left ventricle was thinner (Figure S3H, J) than in wild-type embryos (Figure S3G, I) and at E11.5, in addition to the thin layer of compact myocardium, Mab21l2-deficient embryos had a reduced trabecular myocardium in the left ventricle (Figure 1R) compared to wild-type embryos (Figure 1O), although defects were not grossly observed in the right ventricle of Mab21l2-deficient embryos (Figure 1Q) when they were compared to wild-type embryos (Figure 1N) (After E11.5, defects in the heart region were observed in all Mab21l2-deficient embryos.). At E12.5, defects in the compact and trabecular myocardium were observed not only in the left, but also in the right ventricle of Mab21l2-deficient embryos (Figure 1W, X). Defective morphogenesis of the atrium was not observed in Mab21l2-deficient embryos at E10.5–E12.5 (Figure S3H, 1P, V). No defects in the heart region, including the endocardium and myocardium, were observed at E8.5 (Figure S3A, B) or E9.5 (Figure S3C–F). These results demonstrate that Mab21l2 has an important role in the formation of the trabecular and compact myocardium by E11.5.
BrdU and TUNEL assays demonstrate that the deletion of Mab21l2 causes decreased cell proliferation and increased apoptosis
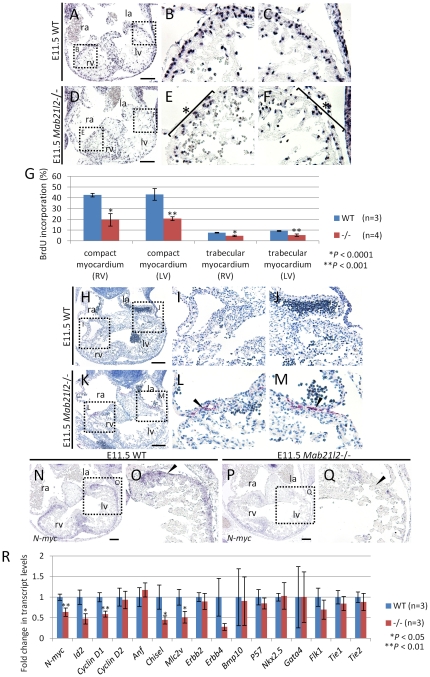
The reduced trabecular myocardium and thin compact myocardium suggest that Mab21l2-deficient embryos have defects in the regulation of cell proliferation and/or apoptosis in these regions after E10.5 or E11.5. The proportions of proliferating cells and apoptotic cells in the heart region were examined using 5-bromo-2′-deoxy-uridine (BrdU) and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assays, respectively. At E10.5, BrdU incorporation was unchanged between wild-type and Mab21l2 mutant hearts, suggesting that the proliferation of myocardial cells was normal (Figure S4). At E11.5, the BrdU assay showed that the number of cells incorporating BrdU in the trabecular and compact myocardium was reduced in Mab21l2 mutant embryos (compact myocardium of right ventricle, <20%, and left ventricle, <20%; WT n = 3, Mab21l2 −/− n = 4; trabecular myocardium of right ventricle, <5%, and left ventricle, <6%; Figure 2D–F) compared to wild-type embryos (Figure 2A–C). BrdU-positive cells were reduced in the ventricular myocardium lying between the atrium and ventricle, indicating that Mab21l2-deficient embryos have decreased cell proliferation in that region. At E10.5, TUNEL-positive cells were not generally detected in the heart region of Mab21l2 mutant embryos (Figure S5D–F). However, in some embryos (1/8 embryos), TUNEL-positive cells were detected in the outermost layer of the myocardium in Mab21l2 mutant embryos (Figure S5G–I). At E11.5, the TUNEL assay showed that TUNEL-positive cells were not detected in the wild-type embryos (Figure 2H–J), but were detected in the outermost layer of the myocardium in Mab21l2-deficient embryos (Figure 2K–M), suggesting that the deletion of Mab21l2 results in apoptotic cell death in that region. These results demonstrate that decreased cell proliferation and increased apoptosis generally occur at around E11.5 and that defective morphogenesis in the trabecular and compact myocardium results from dysregulation of cell proliferation and apoptosis.
Figure 2. Decreased proliferation and increased apoptosis in Mab21l2 mutant embryos.
(A–F) BrdU assay on transverse paraffin sections of E11.5 WT and Mab21l2 mutant (−/−) embryos. BrdU staining shows decreased cell proliferation in the trabecular myocardium and compact myocardium (asterisk) of left (F) and right ventricle (E) in Mab21l2 mutant (D–F) compared to WT embryos (A–C). (G) Quantification of BrdU incorporation. The percentage of BrdU-positive myocardial cells was calculated by dividing the number of BrdU-positive myocardial cells by that of total myocardial cells identified by hematoxylin staining. The values show means of the proportions of BrdU-positive nuclei. Error bars represent the standard deviation and p values were calculated using the two-tailed Student's t test. (H–M) TUNEL assay in transverse paraffin sections of E11.5 embryos. Increased apoptosis is seen in Mab21l2 mutant embryonic hearts (K–M) compared to WT (H–J). Arrowheads indicate TUNEL-positive cells (red). (N–Q) In situ hybridizations on transverse paraffin sections of E11.5 WT and Mab21l2 mutant embryonic hearts for N-myc. N-myc expression was reduced in the compact myocardium of Mab21l2 mutants (P and Q) compared to WT embryos (N and O), especially in the dorsal region of the left ventricle (arrowheads). la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bars represent 100 µm (A, D, H, K) and 50 µm (N and P). (R) Quantitative RT-PCR for genes regulating cell proliferation and apoptosis (N-myc, Id2, Cyclin D1, Cyclin D2), marker genes of myocardial differentiation (Anf, Chisel, Mlc2v), genes essential for the development of the trabecular myocardium (Erbb2, Erbb4, Bmp10), genes essential for heart development (Nkx2.5, Gata4), and endocardial transcripts (Flk1, Tie1, Tie2) normalized to Gapdh expression using RNA from dissected hearts of E11.5 WT and Mab21l2 mutant (−/−) embryos. Error bars represent standard deviation of the mean (WT, n = 3; Mab21l2 (−/−), n = 3). P values were calculated using the unpaired two-tailed Student's t test.
The expression of Mab21l2 is required for the transcription of the gene regulating cell proliferation and apoptosis
Defects in cell proliferation and apoptosis occurred at around E11.5. To understand the molecular basis for these defects, we examined the expression of genes known to regulate these processes using E11.5 embryo samples. N-myc (Mycn - Mouse Genome Informatics), which is involved in the regulation of cell proliferation and apoptosis [25], is expressed in the compact myocardium between E10.5 and E12.5, and the reduced expression of N-myc results in the thinning of the compact myocardium [26], [27], [28], [29], [30]. N-myc expression in the compact myocardium in wild-type and Mab21l2-deficient embryos was examined at E11.5 using in situ hybridization and quantitative RT-PCR. N-myc expression in that region was reduced, especially in the region where cell proliferation was decreased and apoptosis was increased (37%±10% lower) (Figure 2P, Q, R). Decreased expression of Cyclin D1 (Ccnd1 - Mouse Genome Informatics) [31] and Id2 [32], [33], both N-myc target genes, was also observed in the heart region (Id2, 53%±12% lower; Cyclin D1, 42%±7% lower) (Figure 2R), suggesting that Mab21l2 may regulate cell proliferation and apoptosis in the compact myocardium through N-myc expression and via a subset of N-myc target genes, such as Cyclin D1 and Id2.
Nkx2.5 is required for terminal differentiation of cardiomyocytes, including the establishment and/or maintenance of a gene expression program in the ventricles [34], [35], [36]. Gata4 is required for heart tube formation, although Gata4 expression is not essential for specifying the cardiac cell lineages [34], [35], [36]. These genes were normally expressed in Mab21l2 mutant embryos (Figure 2R), suggesting that the deletion of Mab21l2 does not affect cardiac specification and heart tube formation. In Mab21l2-mutant hearts, the expression of differentiation marker genes was decreased, such as the molecular marker for chamber myocardium, Chisel (Smpx - Mouse Genome Informatics) [37], and an early myocardial differentiation marker, Mlc2v (Myl2 - Mouse Genome Informatics) (Chisel, 56%±9% lower; Mlc2v, 49%±13% lower) (Chisel, Figure 2R, Figure S6C, D; Mlc2v, Figure 2R, Figure S6G, H). However, if defects in myocardial differentiation occur, defects in heart development would also occur at much earlier stages. Therefore, decreased expression of myocardial differentiation markers is a secondary effect of decreased cell proliferation. Erbb2 and Erbb4 are expressed in the myocardium, and the signaling pathway activated through the ligand NRG1 and the ERBB2/ERBB4 receptor is essential for the development of the trabecular myocardium [38], [39], [40]. The expression of Erbb2 was unchanged in Mab21l2-deficient embryos (Figure 2R), but that of Erbb4 was reduced in the ventricle of Mab21l2-deficient embryos (73%±8% lower levels; Figure 2R, Figure S6K, L), although this difference could not be statistically confirmed. Bmp10 is expressed after E8.75, and, by E11.5, the expression of Bmp10 is detected primarily in the trabecular region. Bmp10 is required for promoting proliferative activity in myocardial cells through transcriptional regulation of P57 (Cdkn1c - Mouse Genome Informatics) [41], which is an important negative regulator involved in cell cycle exit [42], as well as maintenance of the expression of several important cardiogenic factors, such as Nkx2.5 and Mef2c [41]. However, the expression of Bmp10 and P57 was not altered in Mab21l2 mutant embryos at E11.5 (Figure 2R). The endocardial expression of Flk1 (Kdr - Mouse Genome Informatics), Tie1, and Tie2 (Tek - Mouse Genome Informatics) is required for trabeculation [43], [44], [45], [46]. The expression of these genes was unchanged in Mab21l2 mutants (Figure 2R), suggesting that defective morphogenesis of the trabecular myocardium does not result from defects in endocardial development. These results suggest that reduced expression of Erbb4 has an effect on trabecular development.
Mab21l2 is expressed in the proepicardium and plays a crucial role in the formation of this region and of the epicardium
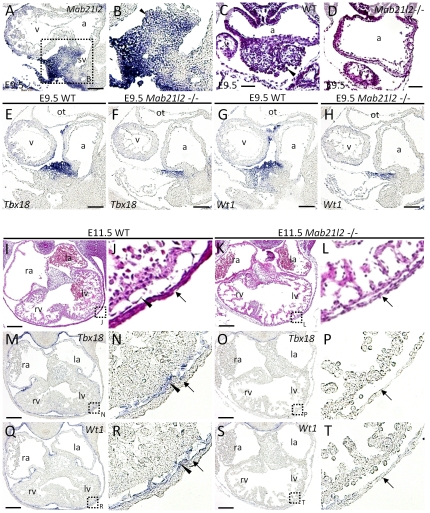
Next, it was examined whether defects occurred in the formation of the tissue adjacent to the ventricular myocardium: the endocardium, which lines the inside of the heart, and the epicardium, which covers the outside. The endocardium is formed in precardiac mesoderm [47], [48], [49], and signaling from the endocardium to the ventricular myocardium is required to initiate the conversion of the myocardial layer of the primitive heart tube into a thickened myocardial wall capable of contraction. The epicardium is formed after E10.5 and is required for optimal myocardial cell proliferation, leading to the formation of the normally contracting myocardium. Mab21l2 expression was not detected in the endocardium or epicardium between E9.5–E12.5 (Figure S1A, B, S2A, B). Mab21l2 was expressed in the proepicardium at E9.5 (Figure 1E, F, 3A, B). To study the effect of Mab21l2 expression on proepicardium formation, we examined whether the proepicardium was normally formed in Mab21l2 mutant embryos. H&E staining showed defective morphogenesis of the proepicardium (Figure 3D). Next, the expression of proepicardial and epicardial marker genes, such as Wt1 [50], and Tbx18 [51] in Mab21l2-deficient embryos was examined. The proepicardium (Tbx18 +, Wt1 + region) was significantly small in Mab21l2 mutants (Tbx18, Figure 3F; Wt1, Figure 3H), demonstrating that defects in proepicardium formation occurred in Mab21l2 mutant embryos. These results suggest that Mab21l2 plays a critical role in the formation of the proepicardium.
Figure 3. Mab21l2 is required for the formation of the proepicardium and the epicardium.
(A,B, E–H, M–T) In situ hybridizations of sagital (A, B, E–H) and transverse (M–T) paraffin sections of E9.5 and E11.5 WT and Mab21l2 mutant embryos for the transcripts indicated. (C, D, I–L) H&E-stained transverse sections of E9.5 and E11.5 WT and Mab21l2 mutant embryos. Mab21l2 expression was detected in the proepicardium (arrowhead) of E9.5 WT embryos (A and B). Compared to WT embryos (C), the proepicardium (arrowhead) was absent in Mab21l2 mutant embryos (D). Expression of the proepicardial and epicardial marker genes, Tbx18, and Wt1, was compared between WT ([E] Tbx18; [G] Wt1), and Mab21l2 mutant embryos ([F] Tbx18; [H] Wt1), showing that the proepicardium is significantly small in Mab21l2 mutant embryos. In E11.5 WT embryos (I and J), the epicardium (arrowhead) occurs between the compact myocardium and the body wall (arrows), but in Mab21l2 mutant embryos (K and L), the epicardium is absent. The expression of epicardial marker genes was detected in E11.5 WT epicardium ([M and N] Tbx18; [Q and R] Wt1), arrowheads), but not in Mab21l2 mutant embryos ([O and P] Tbx18; [S and T] Wt1), between the compact myocardium and the body wall (arrows). a, atrium; v, ventricle; la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle; sv, sinus venosus. Scale bars represent 30 µm (C and D), 50 µm (A, E–H), and 100 µm (I, K, M, O, Q and S).
The defective morphogenesis of the proepicardium could result in defects in the epicardium. H&E staining of heart sections of Mab21l2-deficient embryos suggested that the epicardium was not formed (Figure 3K, L). To confirm the absence of the epicardium, the expression of Tbx18 and Wt1 was examined in Mab21l2 mutant embryos. The expression was not detected around the heart (Tbx18, Figure 3O, P; Wt1, Figure 3S, T), demonstrating that the epicardium is not formed by E11.5. These results suggest that Mab21l2 contributes to the formation of the epicardium through the formation of the proepicardium.
Defective morphogenesis of the epicardium results from reduced expression of α4 integrin and decreased cell proliferation in the small proepicardium
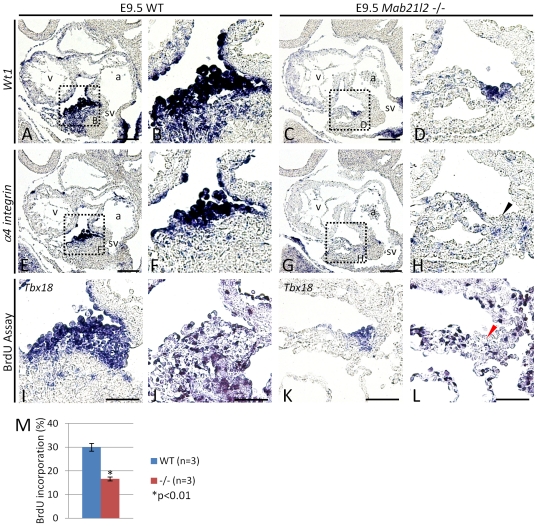
In E9.5 Mab21l2 mutant embryos, the small proepicardium is present, but the epicardium is not formed by E11.5. Among mutants that have defects in epicardium formation, α4 integrin (Itga4 - Mouse Genome Informatics) and Vcam-1 (Vascular cell adhesion molecule-1) mutants have been previously reported [52], [53], [54]. α4 integrin encodes the α4 subunit of α4β1 integrin, a cell adhesion receptor, which binds to fibronectin [55], [56] and VCAM-1 [57], [58]. α4 integrin is expressed in the proepicardium and Vcam-1 is expressed in the myocardium. In α4 integrin and Vcam-1 mutant embryos, the proepicardium is normally formed by E9.5, and by E10.5, the epicardium is also normally formed in α4 integrin mutant embryos, however, by E11.5 the epicardium is absent from both mutant embryos [52], [54], demonstrating that the interaction through α4 integrin and Vcam-1 is required for maintenance of cell adhesion of epicardial cells that have migrated to the surface of the heart. In another α4 integrin mutant line, epicardium formation did not occur by E10.5, suggesting that α4 integrin is also required for proepicardial cell migration from the proepicardium to the surface of the heart, and particularly for proepicardial cell adhesion to the myocardium when proepicardial cells reach this tissue [53]. In the remaining proepicardium and the myocardium, the expression of α4 integrin and Vcam-1 was examined. The expression of Vcam-1 in the myocardium was unchanged (Figure S7A, B), but that of α4 integrin was significantly reduced in the remaining proepicardium (Wt1 + region) (Wt1, Figure 4C, D; α4 integrin, Figure 4G, H). To confirm that the epicardium was absent at E10.5, the expression of Tbx18, and Wt1 was examined in Mab21l2 mutant embryos. Expression was not detected around the heart (Tbx18, Figure S8C, D; Wt1, Figure S8G, H), suggesting that the epicardium is not formed at E10.5. These results suggest that reduced expression of α4 integrin in the remaining proepicardium results in defects of the migration of proepicardial cells, leading to defective morphogenesis of the epicardium by E10.5. Next, cell proliferation and apoptosis in the remaining proepicardium were examined using BrdU and TUNEL assays, respectively. The number of cells incorporating BrdU in the remaining proepicardium expressing Tbx18 was lower in Mab21l2 mutant embryos (<16%; WT n = 3, Mab21l2 −/− n = 3; Figure 4L, M) than in wild-type embryos (Figure 4J, M). However, TUNEL-positive cells were not detected in the remaining proepicardium (data not shown). Taken together, the results suggest that defective morphogenesis of the proepicardium results from dysregulation of cell proliferation in proepicardial cells.
Figure 4. In the remaining proepicardium, α4 integrin expression and cell proliferation are reduced.
(A–H, I and K) In situ hybridizations of sagital paraffin serial sections of E9.5 WT and Mab21l2 mutant embryos for the transcripts indicated. In WT embryos, α4 integrin (F) was expressed in Wt1-positive proepicardial cells (B). In Mab21l2-mutant embryos, the expression of α4 integrin (H; black arrowhead) was significantly reduced in the Wt1-positive proepicardial cells (D). (J and L) BrdU assay on sagital paraffin serial sections of E9.5 from the WT and Mab21l2 mutant (−/−) embryos. BrdU staining shows decreased cell proliferation in the Tbx18-positive proepicardial cells in the Mab21l2 mutant (L; red arrowhead) compared to WT embryos (J). (M) Quantification of BrdU incorporation. The percentage of BrdU-positive proepicardial cells was calculated by dividing the number of BrdU-positive proepicardial cells by that of the total proepicardial cells identified by Tbx18 expression. The values show means of the proportions of BrdU-positive nuclei. Error bars represent the standard deviation and p values were calculated using the two-tailed Student's t test. a, atrium; v, ventricle; sv, sinus venosus. Scale bars represent 50 µm (A, C, E, G) and 30 µm (I–L).
Mab21l2 is expressed in the septum transversum mesenchyme near hepatoblasts and plays an essential role in the formation of the liver
Next, we examined whether Mab21l2 was expressed, not only in the proepicardium, at the anterior of the STM, but also in the rest of the STM. At E8.5, the expression of Mab21l2 was detected in the STM and sinus venosus myocardium (Figure 5A). At E9.5, the STM surrounds the hepatoblasts; therefore, Mab21l2 expression was examined in this region (STM marker: Gata4), and in the hepatoblasts surrounding the liver bud (hepatoblast marker: Prox1). Mab21l2 expression (Figure 5B; Figure 3A, B) overlapped almost completely with Gata4 expression (Figure 5C), but not with Prox1 (Figure 5D), showing that Mab21l2 is expressed in the caudal region of the STM, but not in hepatoblasts. The expression of Mab21l1 was not expressed in the STM.
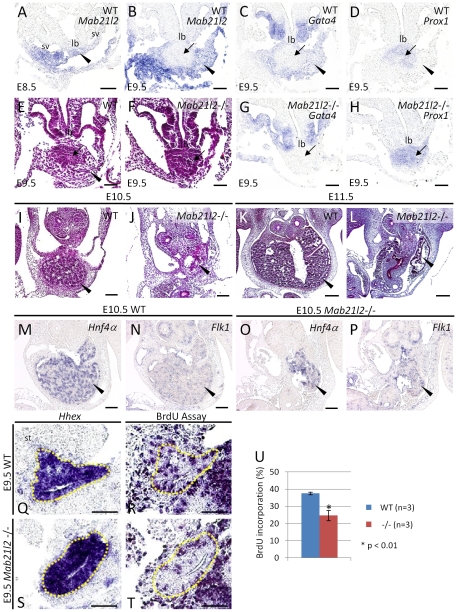
Figure 5. Mab21l2 is required for the formation of the septum transversum mesenchyme near hepatoblasts and for normal liver development.
(A–D, G, H, M–P) In situ hybridizations of transverse paraffin sections of E8.5, E9.5 and E10.5 WT and Mab21l2 mutant embryos, for the transcripts indicated. (Q and S) In situ hybridizations of sagital paraffin sections of E9.5 WT and Mab21l2 mutant embryos for Hhex. (E, F, and I–L) H&E-stained transverse sections of E9.5, E10.5, and E11.5 WT and Mab21l2 mutant embryos. (R and T) BrdU assay on sagital paraffin serial sections of E9.5 from the WT and Mab21l2 mutant (−/−) embryos. (U) Quantification of BrdU incorporation. The percentage of BrdU-positive hepatic cells was calculated by dividing the number of BrdU-positive hepatic cells by that of the total hepatic cells identified according to Hhex expression. The values show means of the proportions of BrdU-positive nuclei. Error bars represent the standard deviation and p values were calculated using the two-tailed Student's t test. Mab21l2 expression was detected in the STM at E8.5 (A). Comparing the expression of Mab21l2 (B), Gata4, an STM marker (C) and Prox1, a hepatoblast marker (D) in E9.5 WT embryos indicated that Mab21l2 is expressed in the STM (arrowheads) near hepatoblasts (arrows) at E9.5. Defective morphogenesis was observed in the region near the liver bud in E9.5 Mab21l2 mutant (F) compared to WT embryos (E). Gata4 was not expressed in this region (G) but Prox1 was expressed (H). Mab21l2 mutant embryos at E10.5 (J) and E11.5 (L) show defective morphogenesis of the liver (arrowheads) compared to WT embryos (I and K). At E10.5, the expression of Hnf4α (hepatoblast marker) and Flk1 (endothelial marker) was detected in Mab21l2 mutant embryos (O and P). BrdU staining shows decreased cell proliferation in the Hhex-positive hepatic region in the Mab21l2 mutant (T) compared to WT embryos (R). lb, liver bud; st, septum transversum mesenchyme; sv, sinus venosus. Scale bar represents 30 µm (A–H, Q–T), 50 µm (I, J, M–P) and 100 µm (K, L).
To study the effect of Mab21l2 expression on the formation of the caudal region of the STM, H&E-stained sections of Mab21l2-deficient embryos were examined. In Mab21l2 mutant embryos, emergence of the liver bud occurred normally, although defective morphogenesis was observed around the liver bud (Figure 5F); however, the region from which the defects originated was unclear. To test whether these defects occurred in the STM or in hepatoblasts, the expression of Gata4 and Prox1 was examined in Mab21l2 mutant embryos. Prox1 expression was detected in hepatoblasts (Figure 5H), but Gata4 was not (Figure 5G). Besides, Tbx18 +, Wt1 + cells (proepicardial cells) were detected in Mab21l2 mutant embryos at E9.5, but Tbx18 −, Wt1 + cells (STM cells except proepicardial cells) were not (Tbx18, Figure 3F; Wt1, Figure 3H). These results show that the STM was absent from the region around the hepatoblasts at E9.5. These results suggest that Mab21l2 plays an essential role in the formation not only of the proepicardium (the STM caudal to the heart), but also of the rest of the STM.
The caudal region of the STM functions as a source of signals for hepatoblast growth and survival, and the inactivation of genes encoding these signaling molecules causes liver hypoplasia [5], and therefore, defects in the STM would be expected to cause dysmorphogenesis of the liver. H&E-stained sections of Mab21l2 mutant embryos at E10.5 and E11.5 suggested that defective morphogenesis of the liver had occurred (Figure 5J, L). To verify whether the remaining tissue was hepatic tissue, the expression of Hnf4α (hepatoblast marker) and Flk1 (endothelial cell marker) was examined. The expression of both genes was detected in Mab21l2 mutant embryos (Figure 5O, P), showing that hepatoblasts, surrounded by endothelial cells, were present in the small liver of Mab21l2 mutant embryos. Next, BrdU and TUNEL assays were performed to examine whether decreased cell proliferation and/or increased apoptosis, respectively, occurred in the hepatic region. The number of cells incorporating BrdU in the hepatic region expressing Hhex was lower in Mab21l2 mutant embryos (<24%; WT n = 3, Mab21l2 −/− n = 3; Figure 5T, U) than in wild-type embryos (<37%; Figure 5R, U). However, no TUNEL-positive cells were detected in the hepatic region (data not shown). Taken together, the results suggest that defective STM morphogenesis results in a decrease in the signals promoting hepatoblast proliferation, leading to a small liver. Next, the expression of the endothelial marker, Flk1, and the hepatoblast markers, Alb, Hhex, and Hnf4a was examined in wild-type and mutant embryos. The expression of Flk1 was unchanged in Mab21l2 mutant embryos (Figure S9E), suggesting that the loss of the basement membrane occurred as normal. The expression of Alb, Hhex and Hnf4a was also unchanged (Figure S9F, H) suggesting that the initial hepatic induction occurred as normal and that defective morphogenesis of the STM does not affect hepatoblast differentiation at E9.5.
Discussion
This study demonstrated that Mab21l2 is expressed in the septum transversum mesenchyme (STM) including the proepicardium, and plays an essential role in the formation of that tissue. The STM is derived from the lateral-plate mesoderm. At E7.5, the presumptive STM is located at the junction between the visceral yolk sac and the amnion. This positional relationship is maintained during neural fold formation and foregut invagination, and, as a result, at E9.0 the STM is located caudal to the heart. Currently, Gata4 is known to be essential for the formation of the proepicardium [59], and the phenotype of Gata4 mutant embryos in proepicardium formation is the same as Mab21l2 mutant embryos. However, the precise molecular mechanisms underlying the formation of the proepicardium remain unknown.
From the anterior surface of the STM, the proepicardium, epicardial progenitor cells migrate to the heart to form the epicardium. In the remaining proepicardium of Mab21l2 mutant embryos, cell proliferation was decreased, suggesting that Mab21l2 contributes to proepicardium formation by promoting the proliferation of proepicardial cells. Although a small proepicardium was present in Mab21l2 mutant embryos, not even transient epicardial formation was observed at E9.5–E10.5, suggesting that defects also occur in the migration of proepicardial cells from the proepicardium to the heart surface in Mab21l2 mutant embryos. Vcam-1 was normally expressed in the myocardium, but the expression of α4 integrin was significantly reduced in the remaining proepicardium (Wt1 + region). α4 integrin is essential for the migration of proepicardial cells onto the heart surface, suggesting that, in Mab21l2 mutant embryos, proepicardial cells that reach the heart surface cannot adhere to the myocardium due to reduced expression of α4-integrin. Thus, during epicardial development, Mab21l2 plays important roles in both proepicardium formation and proepicardial cell migration. Epicardial cells play a crucial role during heart development by providing a source of cells contributing to heart formation, such as cardiac fibroblasts, pericytes and smooth muscle cells [8], [9], [10], [11]. Although beginning to invade the myocardium at E11.5 [60], epicardial cells provide signaling molecules promoting the proliferation of the myocardial cells [12], [13]. Cell proliferation was decreased in E11.5 Mab21l2-mutant embryonic hearts, suggesting that myocardial defects and loss of the epicardium affect cardiomyocyte proliferation. Mice mutant in Wt1, α4integrin or Vcam-1 has defects in epicardium formation, resulting in defects in heart development similar to Mab21l2 mutant embryos, further demonstrating the importance of the epicardium for normal heart development. Taken together, these results suggest that Mab21l2 plays an essential role during heart development through the formation of the proepicardium and the epicardium.
Embryonic lethality in Mab21l2 mutant embryos occurred after E11.5, and the expression of Mab21l2 was detected especially in the left ventricle at E10.5 and E11.5. In E11.5 Mab21l2 mutant embryos, defective morphogenesis was observed not only in the left ventricle, but also in the epicardium. Epicardial cells, surrounding the right and left ventricle, promote cell proliferation of surrounding cardiomyocytes, and it is possible that defects in the epicardium have an effect on cardiomyocyte cell proliferation. If loss of the epicardium has a major influence on defects in the myocardium, these defects would be expected to occur in the right and left ventricle. However, defects were observed only in the left ventricle, suggesting that defective morphogenesis in the left ventricle at E11.5 largely results from loss of function of Mab21l2 in the myocardium. At E12.5, defects in the heart region were observed in the right and left ventricle. At E11.5 and E12.5, the expression of Mab21l2 was not detected in the right ventricle, suggesting that defects in the heart region at E12.5 largely result from loss of the epicardium. The trabecular and compact myocardium are essential for maintaining adequate blood circulation, suggesting that embryonic lethality observed in Mab21l2 mutants at approximately E11.5 may be caused by defective morphogenesis of these regions.
The STM is important not only as a source of progenitors of the epicardium, but also as a source of various signaling components. Prior to and during hepatic induction, inductive signals such as BMPs are expressed in the STM and initiate the liver gene program in the proximal endoderm in conjunction with FGFs from the cardiac mesoderm. In Mab21l2 mutant embryos, marker genes of hepatic induction, such as Alb, Hhex, are normally expressed in the hepatic tissue at E9.5, suggesting that the induction of the liver gene program may normally occur, and at the stage when hepatic induction occurs, the STM may be present. After the induction of liver development, the STM near hepatoblasts, at approximately E9.5, plays an essential role in regulating the growth and survival of hepatoblasts (reviewed in [4], [5]). Reduced cell proliferation was observed in the hepatic region in Mab21l2 mutant embryos at E9.5. Flk1 expression in Mab21l2 mutant embryos suggests that the endothelial basement membrane degrades as expected, and Prox1, which is required for the migration of hepatoblasts into the STM from the liver bud [61], was expressed normally in hepatoblasts in Mab21l2 mutant embryos (Figure 5H), suggesting that hepatoblasts may be clustered in the liver bud in these embryos, because the STM into which hepatoblasts migrate was absent. Other hepatoblast markers, such as Alb, Hhex and Hnf4a, were expressed normally, suggesting that defective morphogenesis of the STM does not affect hepatoblast differentiation at E9.5. Thus, defective liver morphogenesis in Mab21l2 mutant embryos was caused by loss of the signals from the STM that are required for hepatoblast growth. Taken together, these results show that Mab21l2 plays an essential role in liver morphogenesis by mediating the formation of the STM. Mab21l2 is expressed in the STM, not in surrounding hepatoblasts. Loss of function of Mab21l2 results in defective morphogenesis of the STM, leading to defects in cell proliferation of hepatoblasts. These results suggest that Mab21l2 has effects on liver development in a non-cell-autonomous manner.
The STM is a transient tissue that exists during embryogenesis from around E7.5 to E9.5, and is adjacent to the various organs or presumptive organ regions, such as the heart, liver and ventral pancreas. Similar to Mab21l2-deficient mice, Gata4-deficient mice lack the STM, including the proepicardium, at E9.5, but Gata4 mutant mice die by approximately E10.0, and therefore the importance of the STM as a tissue during organogenesis after E10.5 has remained unknown [59], [62]. Fortunately, Mab21l2 mutant mice survive after E10.5, allowing the contribution of the STM to organogenesis after E10.5 to be examined. In this study, we have shown that defects of the STM, including the proepicardium, resulted in the loss of the epicardium and defective morphogenesis of the heart and liver. These results demonstrate that the STM plays essential roles in the morphogenesis of the surrounding organs, such as the heart and liver, although the molecular functions of the MAB21L2 protein remain unclear. To uncover the molecular mechanism underlying STM morphogenesis, it will be necessary to analyze MAB21L2 protein function, which should help to provide a detailed understanding of how the STM functions during the morphogenesis of its surrounding organs.
Materials and Methods
Mice
The generation of mutant mice has been reported previously [22]. Mice were maintained in accordance with protocols approved by the Animal Care and Use Committee of the University of Tokyo.
In situ hybridization
Whole-mount in situ hybridization was performed as described previously [63] at 65°C in 50% formamide containing 5× SSC. Paraffin sections were hybridized in situ at 65°C in 50% formamide, 20 mM Tris-HCl (pH 8.0), 300 mM NaCl, 0.2% Sarkosyl, 1× Denhart's solution, 10% dextran sulfate, and 0.5 mg/ml yeast tRNA. All probes were labeled with digoxigenin using standard procedures. Details for probes will be provided upon request.
Histology
Embryos were dissected in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde in PBS overnight at 4°C. The fixed embryos were dehydrated through a graded alcohol series and embedded in paraffin, sectioned (8 µm thick sections), and stained with hematoxylin and eosin.
Detection of proliferating or apoptotic cells
Pregnant mice were injected intraperitoneally with 3 mg bromodexyuridine (BrdU). Embyros were sacrificed 2 h later and fixed in 4% paraformaldehyde in PBS overnight at 4°C. Sections (8 µm thick) of embryos embedded in paraffin were stained with an anti-BrdU antibody using the BrdU Labeling and Detection Kit II (Roche). TdT-mediated dUTP nick end labeling (TUNEL) analysis was performed as follows. Sections (8 µm thick) of embryos embedded in paraffin were incubated in 3% H2O2 for 15 min, ProK solution for 10 min, and then incubated in TdT reaction solution (0.2 mM fluorescein-12-dUTP (Roche), 0.2 mM dATP, 1 mM CoCl2, 30 mM Tris-HCl (pH 7.5), 140 mM sodium cacodylate, 40 U terminal deoxynucleotidyl transferase (Roche)). Fluorescein was detected with an alkaline phosphatase-conjugated anti-fluorescein antibody (Roche) in a solution containing a phosphatase substrate (Fast Red Tablets, Roche), and counter-stained with hematoxylin.
Quantitative RT-PCR
Whole hearts were dissected from E11.5 embryos. The hearts were disrupted in Sepasol(R)-RNA II Super (Nacalai Tesque) and RNA was purified using standard methods. Synthesis of cDNAs was performed with 150 ng total RNA using M-MuLV Reverse Transcriptase (BioLabs). Quantitative real-time PCR was performed using the SYBR Premix Ex TaqTM (Takara Bio), on a Thermal Cycler Dice Real Time System TP800 (Takara Bio), according to the manufacturer's protocols. All results were normalized to the levels of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA. The primers used in this study were as follows: Anf-Fw, TGTACAGTGCGGTGTCCAAC; Anf-Rv, CCTGCTTCCTCAGTCTGCTC; Bmp10-Fw, CTGAACTGCGGTTGTACACG; Bmp10-Rv, CTCCTCTCCTCCTCGCTACC; Chisel-Fw, CACCGGGAGTTCCTTCTATC; Chisel-Rv, TAGCCCTGCTCTCTGGATTG; Cyclin D1-Fw, GGGACATAGCATCACAGCAG; Cyclin D1-Rv, CCGGAGACTCAGAGCAAATC; Cyclin D2-Fw, ACCGCACACATAGGCTTCTC; Cyclin D2-Rv, ATAACACCTCCTGGGGCTTC; Erbb2-Fw, TCAGCCCCAGAGGATTACAG; Erbb2-Rv, CTGCTCCCAGGATATTCACC; Erbb4-Fw, AACAGCAGTACCGAGCCTTG; Erbb4-Rv, GGATAGACCGCAGGAAGGAG; Flk1-Fw, GGCCGAGTCTGTCTACCTTG; Flk1-Rv, CTTCCTTCCTCCCAGTCCAC; Gata4-Fw, TCTCACTATGGGCACAGCAG; Gata4-Rv, CAGACAGCACTGGATGGATG; Id2-Fw, CCCCAGAACAAGAAGGTGAC; Id2-Rv, ATGCAGGCTGACGATAGTGG; Mlc2v-Fw, TACCCACGGAGAAGAGAAGG; Mlc2v-Rv, CCAGAGCCAAGACTTCCTGT; Nkx2.5-Fw, TTGACGTAGCCTGGTGTCTC; Nkx2.5-Rv, CCCGGTCCTAGTGTGGAATC; N-myc-Fw, AATTGGTCCCCTGTCGAGTC; N-myc-Rv, CACCCAGCATCCCATAAGTC; P57-Fw, GGACGATGGAAGAACTCTGG; P57-Rv, AAAACCGTGGGCTGCTCTAC; Tie1-Fw, CTGCGATGACGAAGTGTACG; Tie1-Rv, CCCAACTGTAGTGCGATCTG; Tie2-Fw, ACCCACTGCCAAGAGATGTG; Tie2-Rv, AGATCCGCACGAGCTGTATG.
Supporting Information
Mab21l2 is expressed in the myocardium. (A–D) In situ hybridizations of Mab21l2 (A and B) and Nkx2.5 (C and D) in transverse serial paraffin sections of WT embryonic hearts at E9.5. Expression of Mab21l2 was detected in the trabecular and compact myocardium expressing Nkx2.5 (a marker for cardiomyocytes). en, endocardium; ot, outflow tract; v, ventricle; t, trabecular myocardium; c, compact myocardium. Scale bar represents 50 µm.
(TIF)
The expression of Mab21l2 at E12.5. (A and B) In situ hybridizations of transverse paraffin sections of E12.5 WT embryonic hearts for Mab21l2. Mab21l2 expression was detected at low levels in the trabecular myocardium (arrow), not in the epicardium (arrowhead) at E12.5 (A and B). la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 100 µm (A).
(TIF)
Defective morphogenesis in the heart region is observed from E10.5. (A–J) H&E-stained transverse sections of E8.5, E9.5 and E10.5 WT and Mab21l2 mutant embryonic hearts. Defects in the heart region were not observed in Mab21l2 mutant embryos (B, D and F) compared to WT embryos (A, C and E) at E8.5 (A and B) and E9.5 (C–F). However, thin compact myocardium was just visible in some Mab21l2 mutant (H and J) compared to WT embryos (G and I) at E10.5 (G–J). cvc, common ventricular chamber; la, left atrium; lv, left ventricle; ot, outflow tract; ra, right atrium; rv, right ventricle. arrows, trabecular myocardium; arrowheads, compact myocardium. Scale bar represents 30 µm (A and B), and 50 µm (C, D, G and H).
(TIF)
At E10.5, cell proliferation in the heart region of the Mab21l2 -mutant is unchanged compared to that of WT embryos. (A–F) BrdU assay on transverse paraffin sections of E10.5 WT and Mab21l2 mutant (−/−) embryos. BrdU staining shows that cell proliferation in the heart region of the Mab21l2-mutant (D–F) is normal, compared to WT embryos (A–C). (G) Quantification of BrdU incorporation. The percentage of BrdU-positive myocardial cells was calculated by dividing the number of BrdU-positive myocardial cells by that of the total myocardial cells identified by hematoxylin staining. The values show means of the proportions of BrdU-positive nuclei. Error bars represent the standard deviation. la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 50 µm.
(TIF)
At E10.5, apoptosis in the heart region is generally unchanged in Mab21l2 -mutant compared to WT embryos. (A–I) TUNEL assay on transverse paraffin sections of E10.5 embryos. (D–F) In Mab21l2 –mutant embryos (D–F), apoptosis was not generally detected in the heart region, but in some Mab21l2-mutant embryos (G–I), increased apoptosis was observed. Arrowhead indicates TUNEL-positive cells (red). lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 50 µm.
(TIF)
Mab21l2 mutants show defective expression of some myocardial differentiation markers and of Erbb4 essential for ventricular trabeculation. (A–L) In situ hybridizations of transverse paraffin sections of E11.5 WT and Mab21l2 mutant embryonic hearts for the transcripts indicated. The expression of Chisel and Mlc2v (cardiomyocyte differentiation markers), was reduced in the myocardium of Mab21l2 mutants (Chisel [C and D]; Mlc2v [G and H]; arrowheads) compared to WT embryos (Chisel [A and B]; Mlc2v [E and F]), especially in the dorsal region of the left ventricle (arrowheads). The expression of Erbb4 (essential for trabecular myocardial development in the heart ventricle) was also reduced in the Mab21l2 mutant myocardium (K and L) compared to WT embryos (I and J). la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 50 µm.
(TIF)
Vcam-1 expression was unchanged in Mab21l2 -mutant myocardium. (A and B) In situ hybridization analysis of Vcam-1 in sagital paraffin sections of E9.5 WT and Mab21l2 mutant embryos. (B) Vcam-1 was normally expressed in the Mab21l2-mutant embryo myocardium compared with WT embryos (A). a, atrium; v, ventricle; sv, sinus venosus. Scale bars represent 50 µm.
(TIF)
Defective morphogenesis of the epicardium occurred by E10.5. (A–H) In situ hybridizations of transverse paraffin sections of E10.5 WT and Mab21l2 mutant embryos for the transcripts indicated. The expression of epicardial marker genes was detected in E10.5 WT epicardium ([A and B] Tbx18; [E and F] Wt1), arrowheads), but not in Mab21l2 mutant embryos ([C and D] Tbx18; [G and H] Wt1) between the compact myocardium and the body wall (arrows). lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 50 µm.
(TIF)
Defective morphogenesis of the STM does not affect the expression of the endothelial marker, Flk1 , or the hepatoblast markers, Alb , Hhex and Hnf4a . (A–H) In situ hybridization of transverse paraffin sections of E9.5 WT and Mab21l2 mutant embryos for the transcripts indicated. Flk1 expression was not altered in Mab21l2 mutant embryos (E), compared to WT embryos (A). The expression of the hepatoblast markers, Alb, Hhex and Hnf4α also remained unaltered in Mab21l2 mutant embryos (F–H), compared to WT embryos (B–D). lb, liver bud; arrowheads, septum transversum mesenchyme. Scale bar represents 30 µm.
(TIF)
Acknowledgments
We appreciate discussion with members of the Takahashi laboratory.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology to N.T. and a Predoctoral Fellowship for Y.S. from JSPS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 6.Manner J, Perez-Pomares JM, Macias D, Munoz-Chapuli R. The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs. 2001;169:89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- 7.Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 8.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Estrada OM, Lettice LA, Essafi A, Guadix JA, Slight J, et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2010;42:89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 12.Lavine KJ, Yu K, White AC, Zhang X, Smith C, et al. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Stuckmann I, Evans S, Lassar AB. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev Biol. 2003;255:334–349. doi: 10.1016/s0012-1606(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 14.Mariani M, Baldessari D, Francisconi S, Viggiano L, Rocchi M, et al. Two murine and human homologs of mab-21, a cell fate determination gene involved in Caenorhabditis elegans neural development. Hum Mol Genet. 1999;8:2397–2406. doi: 10.1093/hmg/8.13.2397. [DOI] [PubMed] [Google Scholar]
- 15.Baird SE, Fitch DH, Kassem IA, Emmons SW. Pattern formation in the nematode epidermis: determination of the arrangement of peripheral sense organs in the C. elegans male tail. Development. 1991;113:515–526. doi: 10.1242/dev.113.2.515. [DOI] [PubMed] [Google Scholar]
- 16.Wong YM, Chow KL. Expression of zebrafish mab21 genes marks the differentiating eye, midbrain and neural tube. Mech Dev. 2002;113:149–152. doi: 10.1016/s0925-4773(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 17.Ho SH, So GM, Chow KL. Postembryonic expression of Caenorhabditis elegans mab-21 and its requirement in sensory ray differentiation. Dev Dyn. 2001;221:422–430. doi: 10.1002/dvdy.1161. [DOI] [PubMed] [Google Scholar]
- 18.Lau GT, Wong OG, Chan PM, Kok KH, Wong RL, et al. Embryonic XMab21l2 expression is required for gastrulation and subsequent neural development. Biochem Biophys Res Commun. 2001;280:1378–1384. doi: 10.1006/bbrc.2001.4290. [DOI] [PubMed] [Google Scholar]
- 19.Mariani M, Corradi A, Baldessari D, Malgaretti N, Pozzoli O, et al. Mab21, the mouse homolog of a C. elegans cell-fate specification gene, participates in cerebellar, midbrain and eye development. Mech Dev. 1998;79:131–135. doi: 10.1016/s0925-4773(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 20.Wong RL, Chan KK, Chow KL. Developmental expression of Mab21l2 during mouse embryogenesis. Mech Dev. 1999;87:185–188. doi: 10.1016/s0925-4773(99)00127-6. [DOI] [PubMed] [Google Scholar]
- 21.Yamada R, Mizutani-Koseki Y, Hasegawa T, Osumi N, Koseki H, et al. Cell-autonomous involvement of Mab21l1 is essential for lens placode development. Development. 2003;130:1759–1770. doi: 10.1242/dev.00399. [DOI] [PubMed] [Google Scholar]
- 22.Yamada R, Mizutani-Koseki Y, Koseki H, Takahashi N. Requirement for Mab21l2 during development of murine retina and ventral body wall. Dev Biol. 2004;274:295–307. doi: 10.1016/j.ydbio.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Wolf LV, Yang Y, Wang J, Xie Q, Braunger B, et al. Identification of pax6-dependent gene regulatory networks in the mouse lens. PLoS One. 2009;4:e4159. doi: 10.1371/journal.pone.0004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunwoodie SL, Rodriguez TA, Beddington RS. Msg1 and Mrg1, founding members of a gene family, show distinct patterns of gene expression during mouse embryogenesis. Mech Dev. 1998;72:27–40. doi: 10.1016/s0925-4773(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 25.Wartiovaara K, Barnabe-Heider F, Miller FD, Kaplan DR. N-myc promotes survival and induces S-phase entry of postmitotic sympathetic neurons. J Neurosci. 2002;22:815–824. doi: 10.1523/JNEUROSCI.22-03-00815.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charron J, Malynn BA, Fisher P, Stewart V, Jeannotte L, et al. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992;6:2248–2257. doi: 10.1101/gad.6.12a.2248. [DOI] [PubMed] [Google Scholar]
- 27.Moens CB, Stanton BR, Parada LF, Rossant J. Defects in heart and lung development in compound heterozygotes for two different targeted mutations at the N-myc locus. Development. 1993;119:485–499. doi: 10.1242/dev.119.2.485. [DOI] [PubMed] [Google Scholar]
- 28.Rustgi AK, Dyson N, Bernards R. Amino-terminal domains of c-myc and N-myc proteins mediate binding to the retinoblastoma gene product. Nature. 1991;352:541–544. doi: 10.1038/352541a0. [DOI] [PubMed] [Google Scholar]
- 29.Sawai S, Shimono A, Wakamatsu Y, Palmes C, Hanaoka K, et al. Defects of embryonic organogenesis resulting from targeted disruption of the N-myc gene in the mouse. Development. 1993;117:1445–1455. doi: 10.1242/dev.117.4.1445. [DOI] [PubMed] [Google Scholar]
- 30.Stanton BR, Perkins AS, Tessarollo L, Sassoon DA, Parada LF. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 1992;6:2235–2247. doi: 10.1101/gad.6.12a.2235. [DOI] [PubMed] [Google Scholar]
- 31.Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- 32.Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2000;407:592–598. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- 33.Lasorella A, Boldrini R, Dominici C, Donfrancesco A, Yokota Y, et al. Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res. 2002;62:301–306. [PubMed] [Google Scholar]
- 34.Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 35.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 36.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 37.Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, et al. Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol. 2000;223:266–278. doi: 10.1006/dbio.2000.9753. [DOI] [PubMed] [Google Scholar]
- 38.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 39.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 40.Lee KF, Simon H, Chen H, Bates B, Hung MC, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Shi S, Acosta L, Li W, Lu J, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochilas LK, Li J, Jin F, Buck CA, Epstein JA. p57Kip2 expression is enhanced during mid-cardiac murine development and is restricted to trabecular myocardium. Pediatr Res. 1999;45:635–642. doi: 10.1203/00006450-199905010-00004. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 44.Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90:9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 46.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 47.Baldwin HS. Early embryonic vascular development. Cardiovasc Res. 1996;31 Spec No:E34–45. [PubMed] [Google Scholar]
- 48.Fishman MC, Chien KR. Fashioning the vertebrate heart: earliest embryonic decisions. Development. 1997;124:2099–2117. doi: 10.1242/dev.124.11.2099. [DOI] [PubMed] [Google Scholar]
- 49.Lough J, Sugi Y. Endoderm and heart development. Dev Dyn. 2000;217:327–342. doi: 10.1002/(SICI)1097-0177(200004)217:4<327::AID-DVDY1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 50.Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- 51.Kraus F, Haenig B, Kispert A. Cloning and expression analysis of the mouse T-box gene Tbx18. Mech Dev. 2001;100:83–86. doi: 10.1016/s0925-4773(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 52.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, et al. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 53.Sengbusch JK, He W, Pinco KA, Yang JT. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol. 2002;157:873–882. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 55.Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- 56.Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 58.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 59.Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Combs MD, Braitsch CM, Lange AW, James JF, Yutzey KE. NFATC1 promotes epicardium-derived cell invasion into myocardium. Development. 2011;138:1747. doi: 10.1242/dev.060996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- 62.Watt AJ, Zhao R, Li J, Duncan SA. Development of the mammalian liver and ventral pancreas is dependent on GATA4. BMC Dev Biol. 2007;7:37. doi: 10.1186/1471-213X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mab21l2 is expressed in the myocardium. (A–D) In situ hybridizations of Mab21l2 (A and B) and Nkx2.5 (C and D) in transverse serial paraffin sections of WT embryonic hearts at E9.5. Expression of Mab21l2 was detected in the trabecular and compact myocardium expressing Nkx2.5 (a marker for cardiomyocytes). en, endocardium; ot, outflow tract; v, ventricle; t, trabecular myocardium; c, compact myocardium. Scale bar represents 50 µm.
(TIF)
The expression of Mab21l2 at E12.5. (A and B) In situ hybridizations of transverse paraffin sections of E12.5 WT embryonic hearts for Mab21l2. Mab21l2 expression was detected at low levels in the trabecular myocardium (arrow), not in the epicardium (arrowhead) at E12.5 (A and B). la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 100 µm (A).
(TIF)
Defective morphogenesis in the heart region is observed from E10.5. (A–J) H&E-stained transverse sections of E8.5, E9.5 and E10.5 WT and Mab21l2 mutant embryonic hearts. Defects in the heart region were not observed in Mab21l2 mutant embryos (B, D and F) compared to WT embryos (A, C and E) at E8.5 (A and B) and E9.5 (C–F). However, thin compact myocardium was just visible in some Mab21l2 mutant (H and J) compared to WT embryos (G and I) at E10.5 (G–J). cvc, common ventricular chamber; la, left atrium; lv, left ventricle; ot, outflow tract; ra, right atrium; rv, right ventricle. arrows, trabecular myocardium; arrowheads, compact myocardium. Scale bar represents 30 µm (A and B), and 50 µm (C, D, G and H).
(TIF)
At E10.5, cell proliferation in the heart region of the Mab21l2 -mutant is unchanged compared to that of WT embryos. (A–F) BrdU assay on transverse paraffin sections of E10.5 WT and Mab21l2 mutant (−/−) embryos. BrdU staining shows that cell proliferation in the heart region of the Mab21l2-mutant (D–F) is normal, compared to WT embryos (A–C). (G) Quantification of BrdU incorporation. The percentage of BrdU-positive myocardial cells was calculated by dividing the number of BrdU-positive myocardial cells by that of the total myocardial cells identified by hematoxylin staining. The values show means of the proportions of BrdU-positive nuclei. Error bars represent the standard deviation. la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 50 µm.
(TIF)
At E10.5, apoptosis in the heart region is generally unchanged in Mab21l2 -mutant compared to WT embryos. (A–I) TUNEL assay on transverse paraffin sections of E10.5 embryos. (D–F) In Mab21l2 –mutant embryos (D–F), apoptosis was not generally detected in the heart region, but in some Mab21l2-mutant embryos (G–I), increased apoptosis was observed. Arrowhead indicates TUNEL-positive cells (red). lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 50 µm.
(TIF)
Mab21l2 mutants show defective expression of some myocardial differentiation markers and of Erbb4 essential for ventricular trabeculation. (A–L) In situ hybridizations of transverse paraffin sections of E11.5 WT and Mab21l2 mutant embryonic hearts for the transcripts indicated. The expression of Chisel and Mlc2v (cardiomyocyte differentiation markers), was reduced in the myocardium of Mab21l2 mutants (Chisel [C and D]; Mlc2v [G and H]; arrowheads) compared to WT embryos (Chisel [A and B]; Mlc2v [E and F]), especially in the dorsal region of the left ventricle (arrowheads). The expression of Erbb4 (essential for trabecular myocardial development in the heart ventricle) was also reduced in the Mab21l2 mutant myocardium (K and L) compared to WT embryos (I and J). la, left atrium; lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 50 µm.
(TIF)
Vcam-1 expression was unchanged in Mab21l2 -mutant myocardium. (A and B) In situ hybridization analysis of Vcam-1 in sagital paraffin sections of E9.5 WT and Mab21l2 mutant embryos. (B) Vcam-1 was normally expressed in the Mab21l2-mutant embryo myocardium compared with WT embryos (A). a, atrium; v, ventricle; sv, sinus venosus. Scale bars represent 50 µm.
(TIF)
Defective morphogenesis of the epicardium occurred by E10.5. (A–H) In situ hybridizations of transverse paraffin sections of E10.5 WT and Mab21l2 mutant embryos for the transcripts indicated. The expression of epicardial marker genes was detected in E10.5 WT epicardium ([A and B] Tbx18; [E and F] Wt1), arrowheads), but not in Mab21l2 mutant embryos ([C and D] Tbx18; [G and H] Wt1) between the compact myocardium and the body wall (arrows). lv, left ventricle; ra, right atrium; rv, right ventricle. Scale bar represents 50 µm.
(TIF)
Defective morphogenesis of the STM does not affect the expression of the endothelial marker, Flk1 , or the hepatoblast markers, Alb , Hhex and Hnf4a . (A–H) In situ hybridization of transverse paraffin sections of E9.5 WT and Mab21l2 mutant embryos for the transcripts indicated. Flk1 expression was not altered in Mab21l2 mutant embryos (E), compared to WT embryos (A). The expression of the hepatoblast markers, Alb, Hhex and Hnf4α also remained unaltered in Mab21l2 mutant embryos (F–H), compared to WT embryos (B–D). lb, liver bud; arrowheads, septum transversum mesenchyme. Scale bar represents 30 µm.
(TIF)