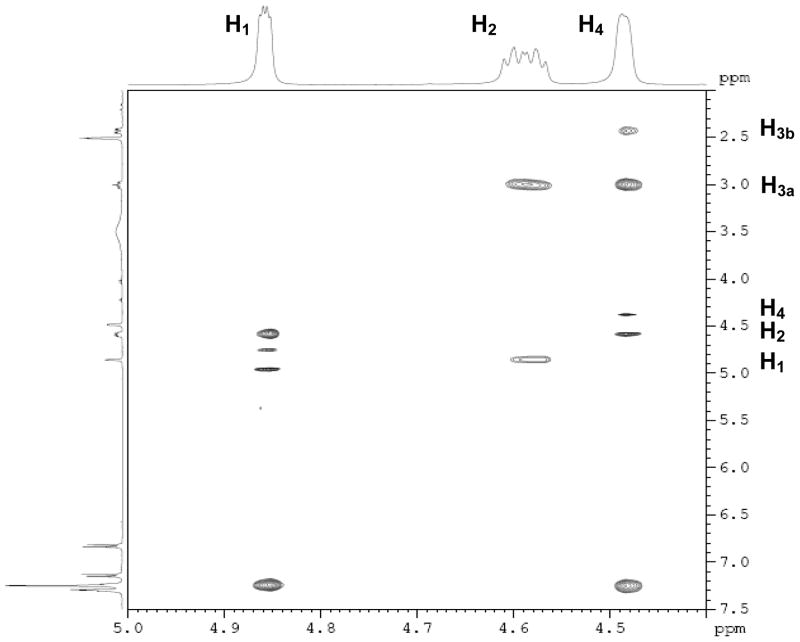
Figure 1.
ROESY-NMR of endo 13. The peaks at δ 4.86 and δ 4.49 are the hydrogen atoms on the bridgehead carbons (H1 and H4), and the peak between them (at δ 4.59) is the hydrogen attached to the carbon bearing the sulfonate group (H2). It is evident that the H2 interacts with the bridgehead hydrogen H1. Since the bridgehead hydrogen is necessarily at an exo position, this interaction indicates that H2 is also at exo position, and, as a result, the sulfonate group is disposed in an endo configuration.