Figure 6.
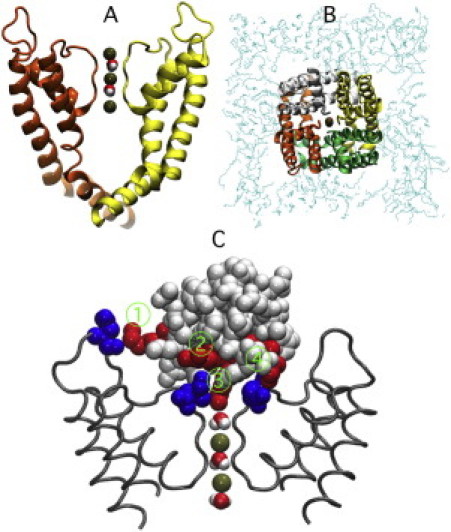
(A) Side (left-hand side) and (B) top (right-hand side) views of the channel system equilibrated for 10 ns with MD simulations. The selectivity filter contains three ions (green) and two water molecules. For clarity, only two of the four subunits are shown for the side view of the channel. (C) Bound configuration for the charybdotoxin molecule to the channel. The arginine and lysine residues on the ChTX molecule that are involved in the binding are shown in red. The corresponding channel aspartate residues are shown in blue. Water and potassium ions inside the selectivity filter are also shown. Close contacts are labeled 1–4: 1), ChTX Lys-31 interacts with channel Asp-355. 2), ChTX Arg-34 interacts with channel Asp-379. 3), ChTX Lys-27 enters the pore. 4), ChTX Arg-25 interacts with channel Asp-379.
