Figure 3.
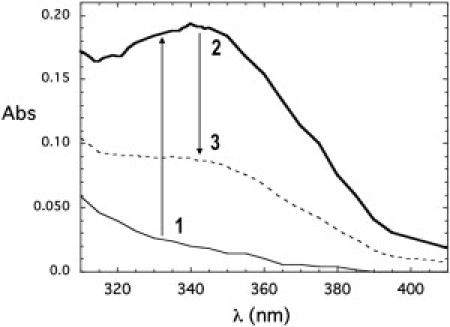
Absorbance spectra illustrating accumulation of the intermediate in the absence of a divalent metal ion. Spectra were obtained at room temperature for 30 mM IPS in 50 mM Tris, pH 7.5, with 300 μM NAD+ and 400 μM EDTA before (thin solid line) and after (bold line) the addition of 5 mM G-6-P and incubation at 85°C for 30 min. The dashed line shows the absorbance when 1 mM MgCl2 was added to the sample, which was then reheated to 85°C for 30 min and then cooled. The arrows and numbers depict the course of the experiment in which EDTA causes accumulation of the intermediate, and addition of metal ions carries the reaction through to completion, reversing the blocking effect of the EDTA.
