Figure 1.
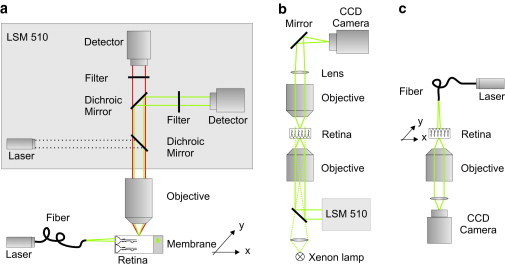
Setups to study the retinal light path. (a) Visualization of the beam path through a retinal cross section. The fluorescent sample, a retinal slice fixed on a nitrocellulose membrane stained with Mitotracker orange, was placed under a water-immersion objective (60×, NA 0.95) of an upright laser-scanning microscope (LSM 510; Zeiss). Confocal images of the evoked fluorescent light (red) were recorded using a confocal channel of the laser-scanning unit (laser 543 nm, green; dichroic mirrors, bandpass filter, detector). An external laser (laser 532 nm, green) was launched into a single mode fiber. The core of the fiber was placed in the focal plane of the objective in front of the vitread surface to illuminate a single Müller cell endfoot. The scattering of the laser light inside the sample (green) was detected by a second channel of the confocal imaging unit (dichroic mirrors, longpass filter, detector). A micropositioning stage (xy stage) moved the sample perpendicular to the light propagation. (b) Bidirectional imaging of retina whole-mount preparations combined with local light-transmission. The retina was spread (photoreceptor side up) onto the bottom of a chamber on the stage of an inverted laser-scanning microscope (LSM 510; Zeiss). The three-dimensional localization of dye-filled Müller cells that were pointed toward the objective (40×, N.A. 1.2) was recorded by confocal detection. Thereafter, the objective was replaced by one with lower numerical aperture (10×, NA 0.3) allowing the illumination of single Müller cell endfeet with laser light under a physiological angle (∼26°). An upright custom-built unit was used to image the opposite (outer) surface of the retina. The laser light transmitted from the photoreceptor outer segments was collected by an objective (63×, N.A. 0.95) and imaged by a convex lens and a plane mirror onto a charge-coupled device chip of a camera. Additionally, the same upright imaging unit allowed the recording of a transmission image of photoreceptor cells by wide-field illumination of the sample with parallel light (Xenon lamp) from below. (c) Spot-light illumination experiments on retinal whole-mount preparations. The retina was spread onto the bottom of a chamber with the photoreceptors pointing toward the objective (63×, NA 1.2) of an inverted microscope. Müller cell endfeet were illuminated by a laser through a single-mode fiber mounted vertically on top of the microscope stage. The glass fiber was moved by a piezo-actuator in steps of 1 μm over a distance of 100 μm. The transmitted laser light was detected by a camera (Zeiss). The fiber had a distance of ∼10 μm from the surface of the tissue.
