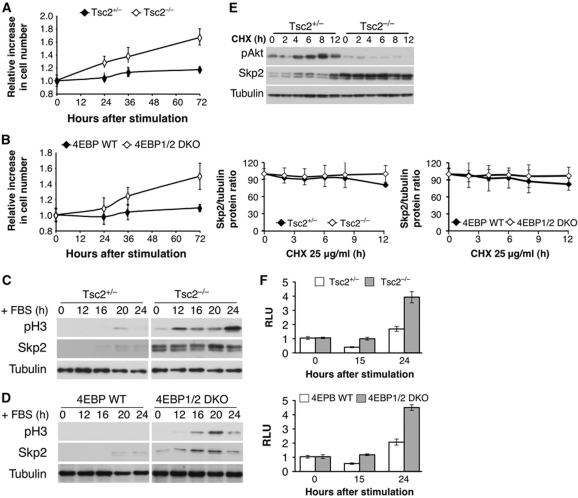
Figure 5.
Tsc2−/− and 4E-BP1/2 DKO cells are capable of exiting contact inhibition and entering into mitosis with concomitant high Skp2 protein expression. (A, B) Two days post confluency, Tsc2+/−, Tsc2−/− MEFs (A) and 4E-BP WT, 4E-BP1/2 DKO MEFs (B) medium was replaced with medium containing 20% FBS, and cell number was measured at 0, 24, 36, and 72 h after stimulation. The relative increase in cell number (compared with time 0) is depicted. Results represent the average ±s.e. of three independent experiments. (C, D) Cell lysates of Tsc2+/−, Tsc2−/− MEFs (C) and 4E-BP WT, 4E-BP1/2 DKO MEFs (D) were prepared at 0, 12, 16, 20, and 24 h post FBS stimulation and subjected to immunoblotting with anti-phospho-H3, Skp2, and tubulin antibodies. (E) Tsc2+/−, Tsc2−/−, 4E-BP WT, and 4E-BP1/2 DKO MEFs were incubated for 0, 2, 4, 6, 8, and 12 h with CHX (25 μg/ml) and cell lysates were prepared and subjected to immunoblotting with anti-Akt, Skp2, and tubulin antibodies. Quantification of at least three experiments was performed and expressed as Skp2 over tubulin protein ratio. Results represent the average ±s.e. of three independent experiments. (F) Tsc2+/−, Tsc2−/−, 4E-BP WT, and 4E-BP1/2 DKO MEFs were co-transfected with Renilla and pGL3 Skp2 5′ UTR luciferase vector as described in Materials and methods. The cells were then grown to confluence and after 2 days medium was replaced with medium containing 20% FBS. At 0, 15, and 24 h post addition of 20% FBS, cells were harvested and analysed with the Dual Luciferase Reporter Assay system. RLU is depicted in the graph. Results are expressed relative to Renilla luciferase luminescence and to time 0 and represent the average ±s.e. of four independent experiments.