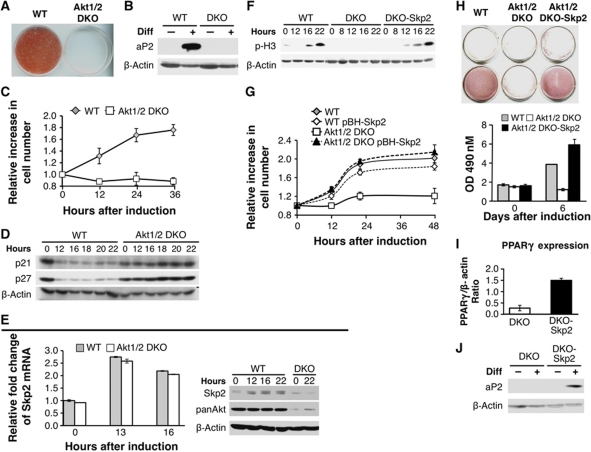
Figure 7.
Akt1/2 DKO preadipocytes fail to undergo MCE and adipogenesis because of the inability to induce Skp2 protein expression. (A) SV40 large T-immortalized preadipocytes, isolated from WT and Akt1/2 DKO mice, were subjected to differentiation in vitro. Differentiation was assessed by Oil-Red-O staining and quantification as described in Materials and methods. (B) Assessment of WT and Akt1/2 DKO preadipocytes by the expression of aP2 using immunoblotting. (C) Akt1/2 DKO preadipocytes fail to undergo MCE. Two days post confluency, cells were induced to differentiate and cell number was measured at 0, 12, 24, and 36 h after induction of differentiation. Results represent the average ±s.e. of three independent experiments. (D) Akt1/2 DKO preadipocytes fail to downregulate p21 and p27 protein expression after induction of differentiation. Two days post confluency, cells were induced to differentiate. Protein extracts were isolated at 0, 12, 16, 18, 20, and 22 h after induction of differentiation and were subjected to immunoblotting using anti-p21 and anti-p27 antibodies. (E) Left panel: Skp2 mRNA levels are induced to the same extent in WT and Akt1/2 DKO preadipocytes immediately after induction of differentiation. Two days post confluency, cells were induced to differentiate and mRNAs were extracted at 0, 13, and 16 h post induction of differentiation. mRNAs were quantified by quantitative RT–PCR. Results represent the average ±s.e. of three independent experiments. Right panel: Skp2 protein is elevated in WT but not in Akt1/2 DKO preadipocytes immediately after induction of differentiation. Two days post confluency, cells were induced to differentiate and proteins were extracted at 0, 12, 16, and 22 h post induction of differentiation. Protein extracts were subjected to immunoblotting, using anti-Skp2, anti-pan Akt, and anti-β actin antibodies. (F) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore entry into mitosis, as measured by the phosphorylation of histone H3, following induction of differentiation. (G) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore MCE. Results represent the average ±s.e. of three independent experiments. (H) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore adipocyte differentiation, as measured by Oil-red-O staining and quantification. Results represent the average ±s.e. of three different experiments. (I) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore the induction of PPARγ expression, as measured by quantitative RT–PCR 5 days after induction of differentiation. Results represent the average ±s.e. of three different experiments. (J) Ectopic expression of Skp2 in Akt1/2 DKO preadipocytes is sufficient to restore the induction of aP2 expression, as measured by immunoblotting, 5 days post induction of differentiation.